Abstract
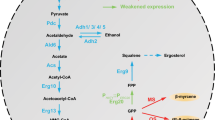
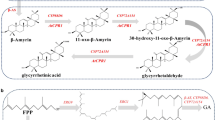
Soyasapogenol B is an oleanane-type pentacyclic triterpene that has various applications in food and healthcare and has a higher biological activity than soyasaponin. Saccharomyces cerevisiae is a potential platform for terpenoid production with mature genetic tools for metabolic pathway manipulation. In this study, we developed a biosynthesis method to produce soyasapogenol B. First, we expressed β-amyrin synthase derived from Glycyrrhiza glabra in S. cerevisiae to generate β-amyrin, as the precursor of soyasapogenol B. Several different types of promoters were then used to regulate the expression of key genes in the mevalonate pathway (MVA), and this subsequently increased the yield of β-amyrin to 17.6 mg/L, 25-fold more than that produced in the original strain L01 (0.68 mg/L). Then, using the β-amyrin-producing strain, we expressed soyasapogenol B synthases from Medicago truncatula (CYP93E2 and CYP72A61V2) and from G. glabra (CYP93E3 and CYP72A566). Soyasapogenol B yields were then optimized by using soyasapogenol B synthases and cytochrome P450 reductase from G. glabra. The most effective soyasapogenol B production strain was used for fermentation, and the yield of soyasapogenol B reached 2.9 mg/L in flask and 8.36 mg/L in a 5-L bioreactor with fed glucose and ethanol. This study demonstrated the heterologous synthesis of soyasapogenol B in S. cerevisiae using the combined expression of CYP93E3 and CYP72A566 in the synthesis pathway, which significantly increased the production of soyasapogenol B and provides a reference method for the biosynthesis of other triterpenes.




Similar content being viewed by others
Data Availability
The data and material are transparent
Code Availability
Not applicable.
References
Lásztity, R., Hidvégi, M., & Bata, Á. (1998). Saponins in food. Food Rev Int, 14(4), 371–390.
Gurfinkel, D. M., & Rao, A. V. (2002). Determination of saponins in legumes by direct densitometry. J Agric Food Chem, 50(3), 426–430.
Lee, S. O., Simons, A. L., Murphy, P. A., & Hendrich, S. (2005). Soyasaponins lowered plasma cholesterol and increased fecal bile acids in female golden Syrian hamsters. Exp Biol Med (Maywood), 230(7), 472–478.
Zha, L. Y., Mao, L. M., Lu, X. C., Deng, H., Ye, J. F., Chu, X. W., Sun, S. X., Luo, H. J., & Luo, H. J. (2011). Anti-inflammatory effect of soyasaponins through suppressing nitric oxide production in LPS-stimulated RAW 264.7 cells by attenuation of NF-κB-mediated nitric oxide synthase expression. Bioorg Med Chem Lett, 21(8), 2415–2418.
Lee, H. J., Lim, S. M., Ko, D. B., Jeong, J. J., Hwang, Y. H., & Kim, D. H. (2017). Soyasapogenol B and genistein attenuate lipopolysaccharide-induced memory impairment in mice by the modulation of NF-κB-mediated BDNF expression. J Agric Food Chem, 65(32), 6877–6885.
Iwamoto, K., Kamo, S., Takada, Y., Ieda, A., Yamashita, T., Sato, T., Zaima, N., Moriyama, T., & Moriyama, T. (2018). Soyasapogenols reduce cellular triglyceride levels in 3T3-L1 mouse adipocyte cells by accelerating triglyceride lipolysis. Biochem Biophys Rep, 16, 44–49.
Rowlands, J. C., Berhow, M. A., & Badger, T. M. (2002). Estrogenic and antiproliferative properties of soy sapogenols in human breast cancer cells in vitro. Food Chem Toxicol, 40(12), 1767–1774.
Zhang, W., & Popovich, D. G. (2008). Effect of soyasapogenol A and soyasapogenol B concentrated extracts on Hep-G2 cell proliferation and apoptosis. J Agric Food Chem, 56(8), 2603–2608.
Wang, L., Wang, J., Zhao, H., Jiang, G., Feng, X., Sui, W., & Liu, H. (2019). Soyasapogenol B exhibits anti-growth and anti-metastatic activities in clear cell renal cell carcinoma. Naunyn-Schmiedebergs Arch Pharmacol, 392(5), 551–563.
Wang, L., Yun, L., Wang, X., Sha, L., Wang, L., Sui, Y., & Zhang, H. (2019). Endoplasmic reticulum stress triggered by Soyasapogenol B promotes apoptosis and autophagy in colorectal cancer. Life Sci, 218, 16–24.
Zhi, L., Song, D., Ma, L., & Feng, T. (2020). Soyasapogenol B attenuates laryngeal carcinoma progression through inducing apoptotic and autophagic cell death. Anat Rec (Hoboken), 303(7), 1851–1858.
Fukushima, E. O., Seki, H., Sawai, S., Suzuki, M., Ohyama, K., Saito, K., & Muranaka, T. (2013). Combinatorial biosynthesis of legume natural and rare triterpenoids in engineered yeast. Plant Cell Physiol, 54(5), 740–749.
Lu, C., Zhang, C., Zhao, F., Li, D., & Lu, W. (2018). Biosynthesis of ursolic acid and oleanolic acid in Saccharomyces cerevisiae. AIChE J, 64(11), 3794–3802.
Wriessnegger, T., & Pichler, H. (2013). Yeast metabolic engineering–targeting sterol metabolism and terpenoid formation. Prog Lipid Res, 52(3), 277–293.
Ma, B., Liu, M., Li, Z. H., Tao, X., Wei, D. Z., & Wang, F. Q. (2019). Significantly enhanced production of patchoulol in metabolically engineered Saccharomyces cerevisiae. J Agric Food Chem, 67(31), 8590–8598.
Ahmed, M. S., Ikram, S., Rasool, A., & Li, C. (2019). Design and construction of short synthetic terminators for Β-amyrin production in Saccharomyces cerevisiae. Biochem Eng J, 146, 105–116.
Zhang, G., Cao, Q., Liu, J., Liu, B., Li, J., & Li, C. (2015). Refactoring β-amyrin synthesis in S accharomyces cerevisiae. AIChE J, 61(10), 3172–3179.
Zhao, C., Xu, T., Liang, Y., Zhao, S., Ren, L., Wang, Q., & Dou, B. (2015). Functional analysis of β-amyrin synthase gene in ginsenoside biosynthesis by RNA interference. Plant Cell Rep, 34(8), 1307–1315.
Ito, R., Masukawa, Y., & Hoshino, T. (2013). Purification, kinetics, inhibitors and CD for recombinant β-amyrin synthase from E uphorbia tirucalli L and functional analysis of the DCTA motif, which is highly conserved among oxidosqualene cyclases. FEBS Journal, 280(5), 1267–1280.
Jin, M. L., Lee, D. Y., Um, Y., Lee, J. H., Park, C. G., Jetter, R., & Kim, O. T. (2014). Isolation and characterization of an oxidosqualene cyclase gene encoding a β-amyrin synthase involved in Polygala tenuifolia Willd. saponin biosynthesis. Plant Cell Rep, 33(3), 511–519.
Jo, H. J., Han, J. Y., Hwang, H. S., & Choi, Y. E. (2017). β-Amyrin synthase (EsBAS) and β-amyrin 28-oxidase (CYP716A244) in oleanane-type triterpene saponin biosynthesis in Eleutherococcus senticosus. Phytochemistry, 135, 53–63.
Ali, M. M., Krishnamurthy, P., El-Hadary, M. H., Kim, J. M., Nawaz, M. A., Yang, S. H., & Chung, G. (2016). Identification and expression profiling of a new β-amyrin synthase gene (GmBAS3) from soybean. Russ J Plant Physiol, 63(3), 383–390.
Chen, H., Liu, Y., Zhang, X., Zhan, X., & Liu, C. (2013). Cloning and characterization of the gene encoding β-amyrin synthase in the glycyrrhizic acid biosynthetic pathway in Glycyrrhiza uralensis. Acta Pharmacol Sin B, 3, 416–424.
El-Hagrassi, A. M., Ali, M. M., Osman, A. F., & Shaaban, M. (2011). Phytochemical investigation and biological studies of Bombax malabaricum flowers. Nat Prod Res, 25(2), 141–151.
Carretero, M. E., López-Pérez, J. L., Abad, M. J., Bermejo, P., Tillet, S., Israel, A., & Noguera-P, B. (2008). Preliminary study of the anti-inflammatory activity of hexane extract and fractions from Bursera simaruba (Linneo) Sarg.(Burseraceae) leaves. J Ethnopharmacol, 116(1), 11–15.
Lin, K. W., Huang, A. M., Tu, H. Y., Lee, L. Y., Wu, C. C., Hour, T. C., Yang, S. C., Pu, Y. S., & Lin, C. N. (2011). Xanthine oxidase inhibitory triterpenoid and phloroglucinol from guttiferaceous plants inhibit growth and induced apoptosis in human NTUB1 cells through a ROS-dependent mechanism. J Agric Food Chem, 59(1), 407–414.
Seki, H., Ohyama, K., Sawai, S., Mizutani, M., Ohnishi, T., Sudo, H., Akashi, T., Aoki, T., Saito, K., & Muranaka, T. (2008). Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci U S A, 105(37), 14204–14209.
Tamura, K., Yoshida, K., Hiraoka, Y., Sakaguchi, D., Chikugo, A., Mochida, K., Kojoma, M., Mitsuda, N., Saito, K., Muranaka, T., & Seki, H. (2018). The basic helix–loop–helix transcription factor gubhlh3 positively regulates soyasaponin biosynthetic genes in Glycyrrhiza uralensis. Plant Cell Physiol, 59(4), 778–791.
Yamamura, Y., & Mabuchi, A. (2020). Functional characterization of NADPH-cytochrome P450 reductase and cinnamic acid 4-hydroxylase encoding genes from Scoparia dulcis L. Bot Stud, 61(1), 6.
Burke, D., Dawson, D., & Stearns, T. (2000). Methods in yeast genetics: a Cold Spring Harbor Laboratory Course Manual (2000th ed.). Cold Spring Harbor Laboratory Press.
Gietz, R. D., Schiestl, R. H., Willems, A. R., & Woods, R. A. (1995). Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast, 11(4), 355–360.
Zeng, B. X., Yao, M. D., Wang, Y., Xiao, W. H., & Yuan, Y. J. (2020). Metabolic engineering of Saccharomyces cerevisiae for enhanced dihydroartemisinic acid production. Front Bioeng Biotechnol, 8, 152.
Li, H., Gao, S., Zhang, S., Zeng, W., & Zhou, J. (2021). Effects of metabolic pathway gene copy numbers on the biosynthesis of (2S)-naringenin in Saccharomyces cerevisiae. J Biotechnol, 325, 119–127.
Schrider, D. R., & Hahn, M. W. (2010). Gene copy-number polymorphism in nature. Proc Biol Sci, 277, 3213–3221.
Yufeng, S., Da Li, H., & Ying, et al. (2019). Cloning and related bioinformatics analysis of new cytochrome NADPH P450 reductase gene in Glycyrrhiza uralensis. Chin Trad Herb Drugs, 50, 1676–1681.
Lan, X., Yuan, W., Wang, M., & Xiao, H. (2019). Efficient biosynthesis of antitumor ganoderic acid HLDOA using a dual tunable system for optimizing the expression of CYP5150L8 and a Ganoderma P450 reductase. Biotechnol Bioeng, 116(12), 3301–3311.
Zhao, F., Bai, P., Liu, T., Li, D., Zhang, X., Lu, W., & Yuan, Y. (2016). Optimization of a cytochrome P450 oxidation system for enhancing protopanaxadiol production in Saccharomyces cerevisiae. Biotechnol Bioeng, 113(8), 1787–1795.
Funding
This work was supported by the Tianjin Natural Science Foundation (NO. 20JCQNJC00740) and the National Key Research and Development Program of China (No. 2019YFA0905100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional Declarations for Articles in Life Science Journals That Report the Results of Studies Involving Humans and/or Animals
This work does not involve humans or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 171 kb)
Rights and permissions
About this article
Cite this article
Li, M., Zhao, M., Wei, P. et al. Biosynthesis of Soyasapogenol B by Engineered Saccharomyces cerevisiae. Appl Biochem Biotechnol 193, 3202–3213 (2021). https://doi.org/10.1007/s12010-021-03599-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03599-5