Abstract
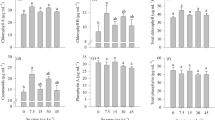
The mungbean plants were grown hydroponically in the absence (control) or presence of 0.1, 0.25, 0.50 and 0.75 ppm selenium (as sodium selenate) for 10 days. The growth of shoots and roots increased with application of selenium with greater extent in shoots. With 0.5 and 0.75 ppm Se levels, the shoot growth was stimulated by 24% to 27% over control, respectively, while the roots showed a corresponding increase of 18–19%, respectively. The shoot-to-root ratio was enhanced significantly with Se application and maximum effects occurred at 0.75 ppm Se. A significant increase was observed in chlorophyll and cellular respiration ability with 0.5 and 0.75 ppm selenium. The increase in growth by selenium was accompanied by elevation of starch, sucrose and reducing sugars. The activity of starch hydrolysing enzymes—amylases and sucrose hydrolysing enzyme—invertase was stimulated significantly with selenium. This was associated with elevation of activities of sucrose synthesising enzymes—sucrose synthase and sucrose phosphate synthase. It was concluded that increase in growth of shoots and roots by application of Se was possibly the result of up-regulation of enzymes of carbohydrate metabolism thus providing energy substrates for enhanced growth.



Similar content being viewed by others
References
Birringer M, Pilawa S, Flohe L (2002) Trends in selenium biochemistry. Nat Prod Rep 19:693–718
Thomson CD (2003) Selenium/Physiology. In: Caballero B, Trugo LC, Finglas PM (eds) Encyclopaedia of Food Science, Food Technology and Nutrition, 2nd edn. Academic Press, London, pp 5117–5124
Ekelund NGA, Danilov RA (2001) The influence of selenium on photosynthesis and “light-enhanced dark respiration” (LEDR) in the flagellate Euglena gracilis after exposure to ultraviolet radiation. Aquat Sci 63:457–465
Brooks RR (1998) Plants that hyperaccumulate heavy metals. Cab International, New York, pp 295–308
Aggarwal M, Sharma S, Kaur N, Pathania D, Bhandhari K, Kaushal N, Kaur R, Singh KJ, Srivastava A, Nayyar H (2010) Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol Trace Elem Res (in press) doi:10.1007/s12011-010-8699-9
Amweg EL, Stuart DL, Weston DP (2003) Comparative bioavailability of selenium to aquatic organisms after biological treatment of agricultural drainage water. Aquat Toxicol 63:13–25
Xue T, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium in senescing lettuce. Plant Soil 237:55–61
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–318
Hartikainen H, Xue T (1999) The promotive effect of selenium on plant growth as triggered by ultraviolet irradiation. J Environ Qual 28:1372–1375
Hawrylak-Nowak B (2009) Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol Trace Elem Res 132:259–269
Yao X, Chu J, Wang G (2009) Effects of selenium on wheat seedlings under drought stress. Biol Trace Elem Res 130:283–290
Pennanen A, Xue T, Hartikainen H (2002) Protective role of selenium in plant subjected to severe UV irradiation stress. J Appl Bot 76:66–76
Turakainen M, Hartikainen H, Seppänen MM (2004) Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J Agric Food Chem 52:5378–5382
Arnon DI (1949) Copper enzyme in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Steponkus PL, Lanphear FO (1967) Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol 42:1423–1426
Nayyar H, Gupta D (2006) Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ Exp Bot 58:106–113
Jones MGK, Outlaw WH Jr, Lowry OH (1977) Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol 60:379–383
Sumner JB, Howell SF (1935) A method for determination of saccharase activity. J Biol Chem 108:51–54
McCready RM, Guggolz J, Silviera V, Owens HS (1950) Determination of starch and amylose in vegetables: application to peas. Anal Chem 29:1156–1158
Dejardin A, Rochat C, Maugenest S, Boutin JP (1997) Purification, characterization and physiological role of sucrose syntase in the pea seed coat (Pisum sativum L.). Planta 201:128–137
Hawker JS, Walker RR, Raffner HP (1976) Invertase and sucrose synthase in flowers. Phytochemistry 15:1411–1443
Huber JLA, Huber SC, Nielsen TH (1989) Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch Biochem Biophys 270:681–690
Shuster L, Gifford RH (1962) Changes in 3-nucleaotidases during the germination of wheat embryo. Arch Biochem Biophys 96:532–540
Nygaard P (1977) Utilization of exogenous carbohydrates for tube growth and starch synthesis in pine pollen suspension culture. Physiol Plant 39:206–210
Angra S, Kaur S, Singh K, Pathania D, Kaur N, Sharma S, Nayyar H (2010) Water-deficit stress during seed filling in contrasting soybean genotypes: association of stress sensitivity with profiles of osmolytes and antioxidants. Int J Agric Res 5:328–345
Moussa HR, El-Fatah A, Ahmed M (2010) Protective role of selenium on development and physiological responses of Vicia faba. Int J Veg Sci 16:174–183
Chen TF, Zheng WJ, Wong YS, Yang FJ (2008) Selenium-induced changes in activities of antioxidant enzymes and content of photosynthetic pigments in Spirulina platensis. J Integr Plant Biol 50:40–48
Padmaja K, Prasad DDK, Prasad ARK (1989) Effect of selenium on chlorophyll biosynthesis in mung bean seedlings. Phytochemistry 128:3321–3324
Germ M, Oswald J (2005) Selenium treatment affected respiratory potential in Eruca sativa. Acta Agric Sloven 85:329–335
Germ M, Kreft I, Stibilj V, Urbanc-Bercic O (2007) Combined effects of selenium and drought on photosynthesis and mitochondrial respiration in potato. Plant Physiol Biochem 45:162–167
Jang JC, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6:1665–1679
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malik, J.A., Kumar, S., Thakur, P. et al. Promotion of Growth in Mungbean (Phaseolus aureus Roxb.) by Selenium is Associated with Stimulation of Carbohydrate Metabolism. Biol Trace Elem Res 143, 530–539 (2011). https://doi.org/10.1007/s12011-010-8872-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8872-1