Abstract
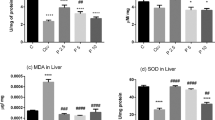
Lead is a persistent environmental pollutant, and its toxicity continues to be a major health problem due to its interference with natural environment. In the present study, we have evaluated the effect of flaxseed oil on lead acetate-mediated hepatic oxidative stress and toxicity in rats. Lead acetate enhanced lipid peroxidation and nitric oxide production in both serum and liver with concomitant reduction in glutathione, catalase, superoxide dismutase, glutathione reductase, glutathione-S-transferase, and glutathione peroxidase activities, these findings were associated with DNA fragmentation. In addition, lead acetate caused liver injury as indicated by histopathological changed of the liver with an elevation in total bilirubin, serum alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, and alkaline phosphatase. Treatment of rats with flaxseed oil resulted in marked improvement in most of the studied parameters as well as histopathological features. On the basis of the above results it can hypothesized that flaxseed oil is a natural product can be protect against lead acetate-mediated hepatic cytotoxicity.


Similar content being viewed by others
References
Skerfving S, Hogstedt C, Welinder H (2007) Broad overview of the history of Swedish occupational health research. Scand J Work Environ Health 33(Suppl 1):6–19
Barbosa F Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ (2005) A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect 113(12):1669–1674
Yu J, Fujishiro H, Miyataka H, Oyama TM, Hasegawa T, Seko Y et al (2009) Dichotomous effects of lead acetate on the expression of metallothionein in the liver and kidney of mice. Biol Pharm Bull 32(6):1037–1042
Bratton GR, Zmudzki J, Bell MC, Warnock LG (1981) Thiamin (vitamin b1) effects on lead intoxication and deposition of lead in tissues: therapeutic potential. Toxicol Appl Pharmacol 59(1):164–172
Flora SJ, Singh S, Tandon SK (1984) Prevention of lead intoxication by vitamin-B complex. Z Gesamte Hyg 30(7):409–411
Traber MG, Packer L (1995) Vitamin E: beyond antioxidant function. Am J Clin Nutr 62(6 Suppl):1501S–1509S
Korsrud GO, Meldrum JB (1988) Effect of diet on the response in rats to lead acetate given orally or in the drinking water. Biol Trace Elem Res 17:167–173
Korsrud GO, Meldrum JB (1988) Effect on blood, liver, and kidney variables of age and of dosing rats with lead acetate orally or via the drinking water. Biol Trace Elem Res 17:151–166
Cohen SL, Moore AM, Ward WE (2005) Flaxseed oil and inflammation-associated bone abnormalities in interleukin-10 knockout mice. J Nutr Biochem 16(6):368–374
Vijaimohan K, Jainu M, Sabitha KE, Subramaniyam S, Anandhan C, Shyamala Devi CS (2006) Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci 79(5):448–454
Kurzer MS, Xu X (1997) Dietary phytoestrogens. Annu Rev Nutr 17:353–381
Jones MM, Cherian MG (1990) The search for chelate antagonists for chronic cadmium intoxication. Toxicology 62(1):1–25
Patrick L (2002) Mercury toxicity and antioxidants: Part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern Med Rev 7(6):456–471
Ito Y, Niiya Y, Kurita H, Shima S, Sarai S (1985) Serum lipid peroxide level and blood superoxide dismutase activity in workers with occupational exposure to lead. Int Arch Occup Environ Health 56(2):119–127
Bhatia AL, Sharma A, Patni S, Sharma AL (2007) Prophylactic effect of flaxseed oil against radiation-induced hepatotoxicity in mice. Phytother Res 21(9):852–859
Tsakiris S, Schulpis KH, Marinou K, Behrakis P (2004) Protective effect of l-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res 49(5):475–479
Watabe M, Masuda Y, Nakajo S, Yoshida T, Kuroiwa Y, Nakaya K (1996) The cooperative interaction of two different signaling pathways in response to bufalin induces apoptosis in human leukemia U937 cells. J Biol Chem 271(24):14067–14072
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Schmidt M, Eisenburg J (1975) Serum bilirubin determination in newborn infants. A new micromethod for the determination of serum of plasma bilirubin in newborn infants. Fortschr Med 93(30):1461–1466
Berkels R, Purol-Schnabel S, Roesen R (2004) Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. Methods Mol Biol 279:1–8
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS (1998) Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J Biol Chem 273(25):15846–15853
Ajayi GO, Adeniyi TT, Babayemi DO (2009) Hepatoprotective and some haematological effects of Allium sativum and vitamin C in lead-exposed wistar rats. Inter J Med Med Sci 1(3):4
Needleman HL (1992) Effects of low levels of lead exposure. Science 256(5055):294–295
Miller JR, Hudson-Edwards KA, Lechler PJ, Preston D, Macklin MG (2004) Heavy metal contamination of water, soil and produce within riverine communities of the Rio Pilcomayo basin, Bolivia. Sci Total Environ 320(2–3):189–209
Pagliara P, Chionna A, Carla EC, Caforio S, Dini L (2003) Lead nitrate and gadolinium chloride administration modify hepatocyte cell surfaces. Cell Tissue Res 312(1):41–48
Patra RC, Swarup D, Dwivedi SK (2001) Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162(2):81–88
El-Nekeety AA, El-Kady AA, Soliman MS, Hassan NS, Abdel-Wahhab MA (2009) Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem Toxicol 47(9):2209–2215
Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A (2005) Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology 206(1):1–15
Adaramoye OA, Osaimoje DO, Akinsanya AM, Nneji CM, Fafunso MA, Ademowo OG (2008) Changes in antioxidant status and biochemical indices after acute administration of artemether, artemether-lumefantrine and halofantrine in rats. Basic Clin Pharmacol Toxicol 102(4):412–418
Sharma V, Kansal L, Sharma A (2009) Prophylactic efficacy of Coriandrum sativum (Coriander) on testis of lead-exposed mice. Biol Trace Elem Res 136(3):337–354
Herman DS, Geraldine M, Venkatesh T (2009) Influence of minerals on lead-induced alterations in liver function in rats exposed to long-term lead exposure. J Hazard Mater 166(2–3):1410–1414
Rahman S, Sultana S (2006) Chemopreventive activity of glycyrrhizin on lead acetate mediated hepatic oxidative stress and its hyperproliferative activity in Wistar rats. Chem Biol Interact 160(1):61–69
Ramesh GT, Jadhav AL (2001) Levels of protein kinase C and nitric oxide synthase activity in rats exposed to sub chronic low level lead. Mol Cell Biochem 223(1–2):27–33
Deneke SM (2000) Thiol-based antioxidants. Curr Top Cell Regul 36:151–180
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539
Anane R, Creppy EE (2001) Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase + catalase and vitamins E and C. Hum Exp Toxicol 20(9):477–481
Lawton LJ, Donaldson WE (1991) Lead-induced tissue fatty acid alterations and lipid peroxidation. Biol Trace Elem Res 28(2):83–97
Sharma A, Sharma V, Kansal L (2009) Therapeutic Effects of Allium sativum on Lead-induced Biochemical changes in Soft tissues of Swiss Albino Mice. Phcog Mag 5:364–371
Sharma V, Sharma A, Kansal L (2010) The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol 48(3):928–936
Sharma V, Pandey D (2010) Protective role of Tinospora cordifolia against lead-induced hepatotoxicity. Toxicol Int 17:12–17
Sharma A, Sharma V, Kansal L (2010) Amelioration of lead induced hepatotoxicity by Allium sativum extracts in Swiss albino mice. Libyan J Med 5:4621
Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU (1999) Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem 202(1–2):91–100
Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342(8878):1007–1011
Prasad K (2005) Hypocholesterolemic and antiatherosclerotic effect of flax lignan complex isolated from flaxseed. Atherosclerosis 179(2):269–275
Thompson LU, Robb P, Serraino M, Cheung F (1991) Mammalian lignan production from various foods. Nutr Cancer 16(1):43–52
Newairy AS, Abdou HM (2009) Protective role of flax lignans against lead acetate induced oxidative damage and hyperlipidemia in rats. Food Chem Toxicol 47(4):813–818
Acknowledgements
This work was gratefully supported by the centre of Excellence for Biodiversity Research, College of Science, King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Moneim, A.E., Dkhil, M.A. & Al-Quraishy, S. The Redox Status in Rats Treated with Flaxseed Oil and Lead-Induced Hepatotoxicity. Biol Trace Elem Res 143, 457–467 (2011). https://doi.org/10.1007/s12011-010-8882-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8882-z