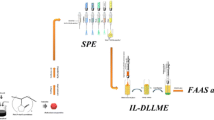
Abstract
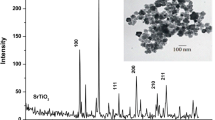
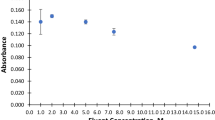
An efficient sorbent based on 2,3-dimercapto-1-propanol immobilized on multi-wall carbon nanotubes (MWCNTs@DMP) was developed for separation/speciation of organic and inorganic lead (alkyl-Pb, Pb2+) in human blood, urine, and water samples by dispersive ionic liquid-suspension-micro-solid phase extraction (DIL-S-μ-SPE). By procedure, the MWCNTs@DMP as solid phase, acetone, and ionic liquid (IL, [HMIM][PF6]) were mixed and injected to 10 mL of the liquid phase at pH = 6.5. After shaking, the Pb(II) was extracted in MWCNTs@DMP and settled down in a conical tube with IL by centrifuging (Pb2+→: SH-SiO2@CNTs). The lead (Pb2+) was back-extracted from sorbent/IL in acidic pH and measured by atom trap atomic absorption spectrometry (AT-AAS). In addition, the organic lead (R-Pb, alkyl lead) converted to Pb(II) and total lead (T-Pb) was determined in the same conditions by UV radiation in 95 °C. Under the optimal conditions, the linear range (9.5–480 μg L−1), LOD (3.2 μg L−1), and enrichment factor (10.4) were obtained (RSD < 5%). The adsorption capacity of the MWCNTs@DMP and MWCNTs was achieved as 191.6 mg g−1 and 25.8 mg g−1, respectively. The method was validated by standard reference materials (SRM 1643d, SRM 955, and SRM 2668), ET-AAS, and ICP-MS analysis in real samples.

Graphical abstract





Similar content being viewed by others
References
World Health Organization (WHO) (2019) Exposure to lead: a major public health concern. https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-19.4.7-eng. Accessed 20 Nov 2019
Adebisi GA, Chowdhury ZZ, Alaba PA (2017) Equilibrium kinetic and thermodynamic studies of lead ion and zinc ion adsorption from aqueous solution onto activated carbon prepared from palm oil mill effluent. J Clean Prod 148:958–968. https://doi.org/10.1016/j.jclepro.2017.02.047
Awual MR (2019) An efficient composite material for selective lead (II) monitoring and removal from wastewater. J Environ Chem Eng 7:103087. https://doi.org/10.1016/j.jece.2019.103087
Latif Wani A, Usmani JA (2015) Lead toxicity a review. Interdiscip Toxicol 8:55–64. https://doi.org/10.1515/intox-2015-0009
Dignam T, Kaufmann RB, LeStourgeon L, Jean Brown M (2019) Control of lead sources in the United States public health progress and current challenges to eliminating lead exposure. J Public Health Manag Pract 25:513–522. https://doi.org/10.1097/PHH.0000000000000889
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead a review with recent updates. Interdiscip Toxicol 5:47–58. https://doi.org/10.2478/v10102-012-0009-2
Kosnett M (2007) Heavy metal intoxication and chelators. Basic and clinical pharmacology, 10th edn. McGraw-Hill, New York, pp 945–957 https://accessmedicine.mhmedical.com/content.aspx?bookid=1193§ionid=69113136
Agency for Toxic Substances and Disease Registry (ATSDR) (2019) Toxicological profile for lead. Atlanta, GA U.S Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=96&tid=22
Fang JY, Wang PW, Huang CH, Hung YY, Pan TL (2014) Evaluation of the hepatotoxic risk caused by lead acetate via skin exposure using a proteomic approach. Proteomics J 14:2588–2599. https://doi.org/10.1002/pmic.201400068
Hanna DA, Hu R, Kim H, Martinez-Guzman O, Torres MP, Reddi AR (2018) Heme bioavailability and signaling in response to stress in yeast cells. J Biol Chem 293:12378–12393. https://doi.org/10.1074/jbc.RA118.002125
Kirberger M, Yang JJ (2008) Structural differences between Pb2+- and Ca2+-binding sites in proteins: implications with respect to toxicity. J Inorg Biochem 102:1901–1909. https://doi.org/10.1016/j.jinorgbio.2008.06.014
Magyar JS, Weng TC, Stern CM, Dye DF, Rous BW, Payne JC, Bridgewater BM, Mijovilovich A, Parkin G, Zaleski JM (2005) Reexamination of lead (II) coordination preferences in sulfur-rich sites: implications for a critical mechanism of lead poisoning. J Am Chem Soc 127:9495–9505. https://doi.org/10.1021/ja0424530
Environmental Protection Agency (USEPA) (2014) Basic Information About Lead in Drinking Water. https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-lead-drinking-water
United States Food and Drug Administration (USFDA) (2020) Elemental impurities guidance for industry. Department of Health and Human Services, Revision 1, p: 1–73. https://www.fda.gov/media/135956/download. Accessed 28 Mar 2017
Atlanta centers for disease control (ACDC) US department of health and human services (2018) national institute for occupational safety and health (NIOSH). Adult blood lead epidemiology and surveillance (ABLES). https://www.cdc.gov/niosh/topics/ables/description.html. Accessed 23 Mar 2017
Arjomandi M, Shirkhanloo H (2019) A review: analytical methods for heavy metals determination in environment and human samples. Anal Method Environ Chem J 2(03):97–126. https://doi.org/10.24200/amecj.v2.i03.73
Shirkhanloo H, Khaligh A, Mousavi HZ, Rashidi A (2015) Graphene oxide-packed micro-column solid-phase extraction combined with flame atomic absorption spectrometry for determination of lead (II) and nickel (II) in water samples. Int J Environ Anal Chem 95:16–32. https://doi.org/10.1080/03067319.2014.983437
Khoshmaram L (2019) Air-assisted liquid–liquid microextraction combined with flame atomic absorption spectrometry for determination of trace Pb in biological and aqueous samples. Int J Environ Anal Chem:1029–0397 (Online) https://www.tandfonline.com/loi/geac20. https://doi.org/10.1080/03067319.2019.1672672
Shamsipur M, Fattahi N, Sadeghi M, Pirsaheb M (2014) Determination of ultra traces of lead in water samples after combined solid-phase extraction–dispersive liquid–liquid microextraction by graphite furnace atomic absorption spectrometry. J Iran Chem Soc 11:249–256. https://doi.org/10.1007/s13738-013-0294-5
Cadorim HR, Schneider M, Hinz J, Luvizon F, Dias AN, Carasek E, Welz B (2019) Effective and high-throughput analytical methodology for the determination of lead and cadmium in water samples by disposable pipette extraction coupled with high-resolution continuum source graphite furnace atomic absorption spectrometry (HR-CS GF AAS). Anal Lett J 52:2133–2149. https://doi.org/10.1080/00032719.2019.1596117
Allafchian A, Mirahmadi-Zare SZ, Gholamian M (2017) Determination of trace lead detection in a sample solution by liquid three-phase microextraction–anodic stripping voltammetry. IEEE Sensors J 17:2856–2862. https://doi.org/10.1109/JSEN.2017.2680450
Mouhamed N, Cheikhou K, Rokhy GEM, Bagha DM, Guèye MDC, Tzedakis T (2018) Determination of lead in water by linear sweep anodic stripping voltammetry (LSASV) at unmodified carbon paste electrode: optimization of operating parameters. Am J Anal Chem 9:171–186. https://doi.org/10.4236/ajac.2018.93015
Eftekhari M, Gheibi M, Akrami M, Iranzad F (2018) Solid-phase extraction of ultra-trace levels of lead using tannic acid-coated graphene oxide as an efficient adsorbent followed by electrothermal atomic absorption spectrometry; response surface methodology–central composite design. New J Chem 42:1159–1168. https://doi.org/10.1039/C7NJ03226A
Martínez D, Grindlay G, Gras L, Mora J (2018) Determination of cadmium and lead in wine samples by means of dispersive liquid–liquid microextraction coupled to electrothermal atomic absorption spectrometry. J Food Compos Anal 67:178–183. https://doi.org/10.1016/j.jfca.2018.01.013
Azimi S, Es’haghi Z (2017) A magnetized nanoparticle based solid-phase extraction procedure followed by inductively coupled plasma atomic emission spectrometry to determine arsenic, lead and cadmium in water, milk, Indian rice and red tea. Bull Environ Contam Toxicol 98:830–836. https://doi.org/10.1007/s00128-017-2068-8
Rehan I, Gondal M, Rehan K (2018) Determination of lead content in drilling fueled soil using laser induced spectral analysis and its cross validation using ICP/OES method. Talanta 182:443–449. https://doi.org/10.1016/j.talanta.2018.02.024
Zhao L, Zhong S, Fang K, Qian Z, Chen J (2012) Determination of cadmium (II), cobalt (II), nickel (II), lead (II), zinc (II), and copper (II) in water samples using dual-cloud point extraction and inductively coupled plasma emission spectrometry. J Hazard Mater 239:206–212. https://doi.org/10.1016/j.jhazmat.2012.08.066
Zolfonoun E (2019) Spectrofluorometric determination of L-tryptophan after preconcentration using multi-walled carbon nanotubes. Anal Method Environ Chem J 2(01):43–48. https://doi.org/10.24200/amecj.v2.i01.43
Fahimirad B, Asghari A (2018) A magnetic graphitic carbon nitride as a new adsorbent for simple separation of Ni (II) ion from foodstuff by ultrasound-assisted magnetic dispersive micro solid-phase extraction method. Anal Method Environ Chem J 1(01):47–56. https://doi.org/10.24200/amecj.v1.i01.36
Gou M, Yarahmadi BB (2019) Separation and determination of lead in human urine and water samples based on thiol functionalized mesoporous silica nanoparticles packed on cartridges by micro column fast micro solid-phase extraction. Anal Method Environ Chem J 2(03):39–50. https://doi.org/10.24200/amecj.v2.i03.72
Shirkhanloo H, Sedighi K, Zavvar Mousavi H (2014) Determination of trace amount of lead in industrial and municipal effluent water samples based on dispersive liquid-liquid extraction. J Mex Chem Soc 58:137–141. https://doi.org/10.29356/jmcs.v58i2.169
Khaligh A, Shirkhanloo H (2019) Food Analysis: Task specific ionic liquids for separation of nickel and cadmium from olive oil samples by thermal ultrasound-assisted dispersive multiphasic microextraction. Anal Method Environ Chem J 2(02):55–64. https://doi.org/10.24200/amecj.v2.i2.64
Wen X, Deng Q, Ji S, Yang S, Peng L (2012) Design of rapidly synergistic cloud point extraction of ultra-trace lead combined with flame atomic absorption spectrometry determination. Microchem J 100:31–35. https://doi.org/10.1016/j.microc.2011.08.005
Shah F, Kazi TG, Afridi HI, Soylak M (2012) Temperature controlled ionic liquid-dispersive liquid phase microextraction for determination of trace lead level in blood samples prior to analysis by flame atomic absorption spectrometry with multivariate optimization. Microchem J 101:5–10. https://doi.org/10.1016/j.microc.2011.09.009
Ghazaghi M, Shirkhanloo H, Mousavi HZ, Rashidi AM (2015) Ultrasound-assisted dispersive solid phase extraction of cadmium (II) and lead (II) using a hybrid nanoadsorbent composed of graphene and the zeolite clinoptilolite. Microchim Acta 182:1263–1272. https://doi.org/10.1007/s00604-015-1446-3
Ghozatlu A, Shariaty-Niassar M (2019) Graphene/CNT hybrid effect on absorption of Mn2+ by activated carbon. Anal Method Environ Chem J 2(02):31–36. https://doi.org/10.24200/amecj.v2.i2.60
Falahnejad M, Mousavi HZ, Shirkhanloo H, Rashidi A (2016) Preconcentration and separation of ultra-trace amounts of lead using ultrasound-assisted cloud point-micro solid phase extraction based on amine functionalized silica aerogel nanoadsorbent. Microchem J 125:236–241. https://doi.org/10.1016/j.microc.2015.11.026
Ghozatlu A (2019) Modification of graphene for speciation of chromium in wastewater samples by suspension solid phase microextraction procedure. Anal Method Environ Chem J 2(03):51–66. https://doi.org/10.24200/amecj.v2.i03.67
Mandlate JS, Soares BM, Seeger TS, Dalla Vecchia P, Mello PA, Flores EM, Duarte FA (2017) Determination of cadmium and lead at sub-ppt level in soft drinks: an efficient combination between dispersive liquid-liquid microextraction and graphite furnace atomic absorption spectrometry. Food Chem 221:907–912. https://doi.org/10.1016/j.foodchem.2016.11.075
Sobhi HR, Mohammadzadeh A, Behbahani M, Esrafili (2019) A Implementation of an ultrasonic assisted dispersive μ-solid phase extraction method for trace analysis of lead in aqueous and urine samples. Microchem J 146:782–788. https://doi.org/10.1016/j.microc.2019.02.008
Davari S, Hosseini F, Shirkhanloo H (2018) Dispersive solid phase microextraction based on amine functionalized bimodal mesoporous silica nanoparticles for separation and determination of calcium ions in chronic kidney disease. Anal Method Environ Chem J 1(01):57–66. https://doi.org/10.24200/amecj.v1.i01.37
Karim MR, Aijaz MO, Alharth NH, Alharbi HF, Al-Mubaddel FS, Awual MR (2019) Composite nanofibers membranes of poly (vinyl alcohol)/chitosan for selective lead (II) and cadmium (II) ions removal from wastewater. Ecotoxic Environ Safe 169:479–486. https://doi.org/10.1016/j.ecoenv.2018.11.049
Kazemi E, Dadfarnia S, HajiShabani AM, Hashemi PS (2017) Synthesis of 2-mercaptobenzothiazole/magnetic nanoparticles modified multi-walled carbon nanotubes for simultaneous solid-phase microextraction of cadmium and lead. Int J Environ Anal Chem 97:743–755. https://doi.org/10.1080/03067319.2017.1353087
Shirkhanloo H, Ahranjani SD (2018) A lead analysis based on amine functionalized bimodal mesoporous silica nanoparticles in human biological samples by ultrasound assisted-ionic liquid trap-micro solid phase extraction. J Pharmaceut Biomed 157:1–9. https://doi.org/10.1016/j.jpba.2018.05.004
Mashkani M, Mehdinia A, Jabbari A, Bide Y, Nabid MR (2018) Preconcentration and extraction of lead ions in vegetable and water samples by N-doped carbon quantum dot conjugated with Fe3O4 as a green and facial adsorbent. Food Chem 239:1019–1026. https://doi.org/10.1016/j.foodchem.2017.07.042
Feis B t, Pilch M, Nycz J (2019) Graphene oxide chemically modified with 5-amino-1, 10-phenanthroline as sorbent for separation and preconcentration of trace amount of lead (II). Microchim Acta 186:91. https://doi.org/10.1007/s00604-018-3213-8
Khajeh M, Heidari ZS, Sanchooli E (2011) Synthesis, characterization and removal of lead from water samples using lead-ion imprinted polymer. Chem Eng J 166:1158–1163. https://doi.org/10.1016/j.cej.2010.12.018
Khajeh M (2011) Response surface modelling of lead pre-concentration from food samples by miniaturised homogenous liquid-liquid solvent extraction: Box-Behnken design. Food Chem 129:1832–1838. https://doi.org/10.1016/j.foodchem.2011.05.123
Khajeh M, Sanchooli E (2011) Silver nanoparticles as a new solid-phase adsorbent and its application to preconcentration and determination of lead from biological samples. Biol Trace Elem Res 143:1856–1864. https://doi.org/10.1007/s12011-011-9013-1
Yasin Y, Ahmad FBH, Ghaffari-Moghaddam M, Khajeh M (2014) Application of a hybrid artificial neural network-genetic algorithm approach to optimize the lead ions removal from aqueous solutions using intercalated tartrate-Mg-Al layered. Environ Nanotechnol Monit Manage 1:2–7. https://doi.org/10.1016/j.enmm.2014.03.001
Saanvi A, Krishnan R, Hassan A, Gupta RK (2020) Fabrication of electrochemical sensor biosystems through hexagonal boron nitride nanosheets for extraction lead in human serum. Anal Methods Environ Chem J 3(01):49–54. https://doi.org/10.24200/amecj.v3.i01.89
Zolfonoun E (2018) Solid phase extraction and determination of indium using multiwalled carbon nanotubes modified with magnetic nanoparticles. Anal Methods Environ Chem J 1(01):5–10. https://doi.org/10.24200/amecj.v1.i01.14
HosseinAbadi MB, Shirkhanloo H, Rakhtshah J (2020) The evaluation of TerphApm@MWCNTs as a novel heterogeneous sorbent for benzene removal from air by solid phase gas extraction. Arab J Chem 13:1741–1751. https://doi.org/10.1016/j.arabjc.2018.01.011
Wang Y, Gao S, Zang X, Li J, Ma J (2012) Graphene-based solid-phase extraction combined with flame atomic absorption spectrometry for a sensitive determination of trace amounts of lead in environmental water and vegetable samples. Anal Chim Acta 716:112–118. https://doi.org/10.1016/j.aca.2011.12.007
Ezoddin M, Shemirani F, Abdi K, Saghezchi MK, Jamali M (2010) Application of modified nano-alumina as a solid phase extraction sorbent for the preconcentration of Cd and Pb in water and herbal samples prior to flame atomic absorption spectrometry determination. J Hazard Mater 178:900–905. https://doi.org/10.1016/j.jhazmat.2010.02.023
Duran A, Tuzen M, Soylak M (2009) Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J Hazard Mater 169:466–471. https://doi.org/10.1016/j.jhazmat.2009.03.119
Krawczyk M, Jeszka-Skowron M (2016) Multiwalled carbon nanotubes as solid sorbent in dispersive micro solid-phase extraction for the sequential determination of cadmium and lead in water samples. Microchem J 126:296–301. https://doi.org/10.1016/j.microc.2015.12.027
Acknowledgments
The authors wish to thank Semnan University Research Council, Semnan, Iran, Iranian Petroleum Industry Health Research Institute (IPIHRI), and the Iranian Research Institute of Petroleum Industry (RIPI) for supporting this work. The authors wish to thank the workers for their kindness and voluntary participation in this study.
Funding
This study was supported by Semnan University by a grant
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of Semnan University (ECSU, Project No. 8051127-01). Before starting, the goals and stages of the study were explained to the participants and they were asked to sign the informed consent form. The participants were assured that they could leave the study whenever they wanted.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 309 kb)
Rights and permissions
About this article
Cite this article
Esmaeili, N., Rakhtshah, J., Kolvari, E. et al. Rapid Speciation of Lead in Human Blood and Urine Samples Based on MWCNTs@DMP by Dispersive Ionic Liquid-Suspension-Micro-Solid Phase Extraction. Biol Trace Elem Res 199, 2496–2507 (2021). https://doi.org/10.1007/s12011-020-02382-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02382-7