Abstract
In recent years, studies investigating the protective effect of hydrogen-rich water (HRW) against different diseases and the toxicity of some substances have attracted increasing attention. Here, we assessed the effects of hydrogen-rich water on different nickel-induced toxic responses (reactive oxygen species (ROS), tumor necrosis factor-alpha (TNF-α), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) of stress responses, histopathological changes) and cocoon production in earthworm model. Earthworms were randomly divided into two main groups: water (W) group including control (CW: ultrapure water), 10 (10W), 200 (200W), and 500 (500W), and hydrogen-rich ultrapure water (HRW) group including control (CHRW: hydrogen-rich ultrapure water), 10 (10HRW), 200 (200HRW), and 500 (500HRW) mg of nickel chloride kg−1 soil for 14 days. We found that cocoon production was less affected by the nickel exposure of earthworms in the 500HRW group compared to the 500W group. The ROS levels in 200HRW and 500HRW groups were less than that of 200W and 500W, respectively. The epithelial degeneration, epithelial necrosis, and necrosis in muscle fibers in tissues of earthworm were less damaged in 200HRW and 500HRW groups compared to 200W and 500W, respectively. HRW groups significantly reduced the expression of 8-OHdG induced by nickel exposure and inflammatory cytokine response including TNF-α. The study showed that hydrogen-rich water could alleviate the toxic effects of nickel-induced oxidative and inflammatory damages in earthworms. The HRW treatment known for its cheap and eco-friendly propertıes without any negative effects on the ecosystem can be used as a green method for alleviating the toxification effects of heavy metals in contaminated soil and increasing cocoon production of earthworms.
Graphical abstract





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Duffus JH (2002) “heavy metals” - a meaningless term? (IUPAC technical report). Pure Appl Chem 74:793–807. https://doi.org/10.1351/pac200274050793
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Molecular, clinical and environmental toxicicology. Environ Toxicol 3
Mahboob S, Alkkahem Al-Balwai HF, Al-Misned F et al (2014) A study on the accumulation of nine heavy metals in some important fish species from a natural reservoir in Riyadh, Saudi Arabia. Toxicol Environ Chem 96:783–798. https://doi.org/10.1080/02772248.2014.957485
Slaveykova VI, Cheloni G (2018) Preface: special issue on environmental toxicology of trace metals. Environments - MDPI 5:138. https://doi.org/10.3390/environments5120138
Eisler R (1998) Nickel hazards to fish, wildlife, and invertebrates: a synoptic review. Contaminant Hazard Reviews Report 34. USGS/BRD/ BSR-1998–001. U.S. Geological Survey, Washington DC
Ma J, Pan G, Wan H et al (2004) Investigation on heavy metal pollution in a typical area of the Pearl River Delta. Chin J Soil Sci 35:636–638
Brieger K, Schiavone S, Miller FJ, Krause KH (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659. https://doi.org/10.4414/smw.2012.13659
Grivennikova VG, Vinogradov AD (2013) Mitochondrial production of reactive oxygen species. Biochem Mosc 78:1490–1511. https://doi.org/10.1134/S0006297913130087
Matés JM, Segura JA, Alonso FJ, Márquez J (2010) Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med 49:1328–1341. https://doi.org/10.1016/j.freeradbiomed.2010.07.028
Gomes Muniz FWM, Nogueira SB, Vasconcelos Mendes FL et al (2015) The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: a systematic review. Arch Oral Biol 60:1203–1214. https://doi.org/10.1016/j.archoralbio.2015.05.007
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their ımplication in various diseases. Indian J Clin Biochem 30:11–26. https://doi.org/10.1007/s12291-014-0446-0
Li H, Yin Y, Liu J et al (2021) Hydrogen-rich water attenuates the radiotoxicity induced by tritium exposure in vitro and in vivo. J Radiat Res 62:34–45. https://doi.org/10.1093/jrr/rraa104
Xiao L, Miwa N (2017) Hydrogen-rich water achieves cytoprotection from oxidative stress injury in human gingival fibroblasts in culture or 3D-tissue equivalents, and wound-healing promotion, together with ROS-scavenging and relief from glutathione diminishment. Hum Cell 30:72–87. https://doi.org/10.1007/s13577-016-0150-x
Wang G, Xia X, Yang J et al (2020) Exploring the bioavailability of nickel in a soil system: physiological and histopathological toxicity study to the earthworms (Eisenia fetida). J Hazard Mater 383:121169. https://doi.org/10.1016/J.JHAZMAT.2019.121169
Cadet J, Delatour T, Douki T et al (1999) Hydroxyl radicals and DNA base damage. Mutat Res Fundam Mol Mech Mutagen 424:9–21. https://doi.org/10.1016/S0027-5107(99)00004-4
Cheng KC, Cahill DS, Kasai H et al (1992) 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G → T and A → C substitutions. J Biol Chem 267:166–172. https://doi.org/10.1016/s0021-9258(18)48474-8
Wu D, Liu B, Yin J et al (2017) Detection of 8-hydroxydeoxyguanosine (8-OHdG) as a biomarker of oxidative damage in peripheral leukocyte DNA by UHPLC–MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 1064:1–6. https://doi.org/10.1016/j.jchromb.2017.08.033
Dally H, Hartwig A (1997) Induction and repair inhibition of oxidative DNA damage by nickel(II) and cadmium(II) in mammalian cells. Carcinogenesis 18:1021–1026. https://doi.org/10.1093/carcin/18.5.1021
Dinarello CA (2007) Historical insights into cytokines. Eur J Immunol 37:S34–S45. https://doi.org/10.1002/eji.200737772
Feldmann M (2008) Many cytokines are very useful therapeutic targets in disease. J Clin Invest 118:3533–3536. https://doi.org/10.1172/JCI37346.logic
Baud V, Karin M, Karin M (2001) Signal transduction by TNF and its relatives. Trends Cell Biol 11:372–377
Carswell EA, Old LJ, Kassel RL et al (1975) An endotoxin-induced serum factor that causes necrosis of tumors (activated macrophage). Immunology 72:3666–3670
Agrawal NK (2014) Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes 5:697. https://doi.org/10.4239/wjd.v5.i5.697
Wang H, Li J, Gai Z, Kullak-Ublick GA, Liu Z (2017) TNF-α deficiency prevents renal ınflammation and oxidative stress in obese mice. Kidney Blood Press Res 42:416–427. https://doi.org/10.1159/000478869
ElMahdy MK, Helal MG, Ebrahim TM (2020) Potential anti-inflammatory effect of dapagliflozin in HCHF diet- induced fatty liver degeneration through inhibition of TNF-α, IL-1β, and IL-18 in rat liver. Int Immunopharmacol 86:106730. https://doi.org/10.1016/j.intimp.2020.106730
Iwai-Shimada M, Takahashi T, Kim MS et al (2016) Methylmercury induces the expression of TNF-α selectively in the brain of mice. Sci Rep 6:1–8. https://doi.org/10.1038/srep38294
Bonaventura P, Lamboux A, Albarède F, Miossec P (2018) Differential effects of TNF-α and IL-1β on the control of metal metabolism and cadmium-induced cell death in chronic inflammation. PLoS ONE 13:e0196285. https://doi.org/10.1371/journal.pone.0196285
López-Vanegas NC, Hernández G, Maldonado-Vega M, Calderón-Salinas JV (2020) Leukocyte apoptosis, TNF-α concentration and oxidative damage in lead-exposed workers. Toxicol Appl Pharmacol 391:114901. https://doi.org/10.1016/j.taap.2020.114901
Ding J, Huang Y, Ning B et al (2009) TNF-α Induction by nickel compounds is specific through ERKs/AP-1- dependent pathway in human bronchial epithelial cells Jin. Curr Cancer Drug Targets 9:81–90. https://doi.org/10.2174/156800909787313995
Huang H, Zhu J, Li Y et al (2016) Upregulation of SQSTM1/p62 contributes to nickel-induced malignant transformation of human bronchial epithelial cells. Autophagy 12:1687–1703. https://doi.org/10.1080/15548627.2016.1196313
Xin R, Pan YL, Wang Y et al (2019) Nickel-refining fumes induce NLRP3 activation dependent on mitochondrial damage and ROS production in Beas-2B cells. Arch Biochem Biophys 676:108148. https://doi.org/10.1016/j.abb.2019.108148
Xie K, Liu L, Yu Y, Wang G (2014) Hydrogen gas presents a promising therapeutic strategy for sepsis. BioMed Res 2014. https://doi.org/10.1155/2014/807635
Dixon BJ, Tang J, Zhang JH (2013) The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res 3:1–12. https://doi.org/10.1186/2045-9912-3-10
Lian N, Shen M, Zhang K et al (2021) Drinking hydrogen-rich water alleviates chemotherapy-induced neuropathic pain through the regulation of gut microbiota. J Pain Res 14:681. https://doi.org/10.2147/JPR.S288289
Zheng M, Yu H, Xue Y et al (2021) The protective effect of hydrogen-rich water on rats with type 2 diabetes mellitus. Mol Cell Biochem 476:3089–3097. https://doi.org/10.1007/s11010-021-04145-x
Nakata K, Yamashita N, Noda Y, Ohsawa I (2015) Stimulation of human damaged sperm motility with hydrogen molecule. Med Gas Res 5:1–8. https://doi.org/10.1186/s13618-014-0023-x
He X, Wang SY, Yin CH et al (2016) Hydrogen-rich water exerting a protective effect on ovarian reserve function in a mouse model of immune premature ovarian failure induced by zona pellucida 3. Chin Med J 129:2331. https://doi.org/10.4103/0366-6999.190668
Ku JY, Park MJ, Park HJ et al (2020) Combination of Korean Red Ginseng extract and hydrogen-rich water improves spermatogenesis and sperm motility in male mice. Chin J Integr Med 1–9. https://doi.org/10.1007/s11655-019-3047-1
Gharib B, Hanna S, Abdallahi OMS et al (2001) Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. Comptes Rendus de l’Académie des Sciences - Series III - Sciences de la Vie 324:719–724. https://doi.org/10.1016/S0764-4469(01)01350-6
Li J, Wang C, Zhang JH et al (2010) Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res 1328:152–161
Zhang Y, Sun Q, He B et al (2011) Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol 148:91–95. https://doi.org/10.1016/j.ijcard.2010.08.058
Hu Z, Wu B, Meng F et al (2017) Impact of molecular hydrogen treatments on the innate immune activity and survival of zebrafish (Danio rerio) challenged with Aeromonas hydrophila. Fish Shellfish Immunol 67:554–560. https://doi.org/10.1016/j.fsi.2017.05.066
Köktürk M, Atalar MN, Odunkıran A et al (2021) Evaluation of the hydrogen-rich water alleviation effect on mercury toxicity in earthworms using ATR-FTIR and LC-ESI-MS/MS Spectroscopy. Environ Sci Pollut Res under revision
Ryu J, Kim MJ, Lee JH (2019) Extraction of green tea phenolics using water bubbled with gases. J Food Sci 84:1308–1314. https://doi.org/10.1111/1750-3841.14606
OECD (1984) Organization for Economical Cooperation and Development guideline for testing of chemicals, No. 207, Earthworm Acute Toxicity, OECD, Paris
Xu D, Li C, Wen Y, Liu W (2013) Antioxidant defense system responses and DNA damage of earthworms exposed to Perfluorooctane sulfonate (PFOS). Environ Pollut 174:121–127
Nakashima T, Okada T, Asahi J et al (2008) 8-Hydroxydeoxyguanosine generated in the earthworm Eisenia fetida grown in metal-containing soil. Mutat Res Genet Toxicol Environ Mutagen 654:138–144. https://doi.org/10.1016/j.mrgentox.2008.05.011
Lawler JM, Song W, Demaree SR (2003) Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radical Biol Med 35:9–16. https://doi.org/10.1016/S0891-5849(03)00186-2
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Topal A, Alak G, Ozkaraca M et al (2017) Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere 175:186–191. https://doi.org/10.1016/j.chemosphere.2017.02.047
Türkoğlu M, Baran A, Sulukan E et al (2021) The potential effect mechanism of high-fat and high-carbohydrate diet-induced obesity on anxiety and offspring of zebrafish. Eat Weight Disord 1–15. https://doi.org/10.1007/s40519-021-01140-5
Yan Z, Wang B, Xie D et al (2011) Uptake and toxicity of spiked nickel to earthworm Eisenia fetida in a range of Chinese soils. Environ Toxicol Chem 30:2586–2593. https://doi.org/10.1002/etc.657
Kong L, Gao X, Zhu J et al (2017) Reproductive toxicity induced by nickel nanoparticles in Caenorhabditis elegans. Environ Toxicol 32:1530–1538. https://doi.org/10.1002/TOX.22373
Sun H, Wu W, Guo J et al (2016) Effects of nickel exposure on testicular function, oxidative stress, and male reproductive dysfunction in Spodoptera litura Fabricius. Chemosphere 148:178–187. https://doi.org/10.1016/j.chemosphere.2015.10.068
Rizvi A, Parveen S, Khan S, Naseem I (2020) Nickel toxicology with reference to male molecular reproductive physiology. Reprod Biol 20:3–8. https://doi.org/10.1016/j.repbio.2019.11.005
Ku JY, Park MJ, Park HJ et al (2020) Combination of Korean Red Ginseng extract and hydrogen-rich water ımproves spermatogenesis and sperm motility in male mice. Chin J Integr Med 26:361–369. https://doi.org/10.1007/s11655-019-3047-1
Iuchi K, Imoto A, Kamimura N et al (2016) Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators OPEN. Nat Publ Group 6:1–12. https://doi.org/10.1038/srep18971
Wen S, Liu C, Wang Y et al (2021) Oxidative stress and DNA damage in earthworm (Eisenia fetida) induced by triflumezopyrim exposure. Chemosphere 264:128499. https://doi.org/10.1016/j.chemosphere.2020.128499
Yang Y, Zhu Y, Xi X (2018) Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species. Oncol Lett 16:2771–2776. https://doi.org/10.3892/ol.2018.9023
Ohsawa I, Ishikawa M, Takahashi K et al (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694. https://doi.org/10.1038/nm1577
Genchi G, Carocci A, Lauria G et al (2020) Nickel: human health and environmental toxicology. Int J Environ Res Public Health 17:679
Ohta S (2012) Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta (BBA) Gen Subj 1820:586–594
Xie K, Yu Y, Pei Y et al (2010) Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock 34:90–97
Tao G, Song G, Qin S (2019) Molecular hydrogen: current knowledge on mechanism in alleviating free radical damage and diseases. Acta Biochim Biophys Sin 51:1189–1197
Sauer H, Wartenberg M, Hescheler J (2001) Reactive oxygen species as ıntracellular messengers during cell growth and differentiation. Cell Physiol Biochem 11:173–186. https://doi.org/10.1159/000047804
Xiao L, Miwa N (2021) Hydrogen nano-bubble water suppresses ROS generation, adipogenesis, and interleukin-6 secretion in hydrogen-peroxide- or PMA-stimulated adipocytes and three-dimensional subcutaneous adipose equivalents. Cells 10:626. https://doi.org/10.3390/cells10030626
Babu D, Leclercq G, Motterlini R, Lefebvre RA (2017) Differential effects of CORM-2 and CORM-401 in murine intestinal epithelial MODE-K cells under oxidative stress. Front Pharmacol 8:31. https://doi.org/10.3389/fphar.2017.00031
Gomes SIL, Roca CP, Scott-Fordsmand JJ, Amorim MJB (2019) High-throughput transcriptomics: insights into the pathways involved in (nano) nickel toxicity in a key invertebrate test species. Environ Pollut 245:131–140. https://doi.org/10.1016/j.envpol.2018.10.123
Yang Y, Liu PY, Bao W et al (2020) Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer 20:1–19. https://doi.org/10.1186/s12885-019-6491-6
Sim M, Kim CS, Shon WJ et al (2020) Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: a randomized, double-blind, controlled trial. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-68930-2
Chow MT, Duret H, Andrews DM et al (2014) Type I NKT-cell-mediated TNF-α is a positive regulator of NLRP3 inflammasome priming. Eur J Immunol 44:2111–2120. https://doi.org/10.1002/eji.201344329
Bauernfeind F, Niepmann S, Knolle PA, Hornung V (2016) Aging-associated TNF production primes inflammasome activation and NLRP3-related metabolic disturbances. J Immunol 197:2900–2908. https://doi.org/10.4049/jimmunol.1501336
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336. https://doi.org/10.1016/0891-5849(94)00159-H
Jing H, Zhang Q, Li S, Gao X, jiao, (2020) Pb exposure triggers MAPK-dependent inflammation by activating oxidative stress and miRNA-155 expression in carp head kidney. Fish Shellfish Immunol 106:219–227. https://doi.org/10.1016/j.fsi.2020.08.015
Renu K, Chakraborty R, Myakala H et al (2021) Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) - induced hepatotoxicity – A review. Chemosphere 271:129735. https://doi.org/10.1016/j.chemosphere.2021.129735
Hu J, Liu J, Li J et al (2021) Metal contamination, bioaccumulation, ROS generation, and epigenotoxicity influences on zebrafish exposed to river water polluted by mining activities. J Hazard Mater 405:124150. https://doi.org/10.1016/j.jhazmat.2020.124150
Lupescu A, Jilani K, Zelenak C et al (2012) Hexavalent chromium-induced erythrocyte membrane phospholipid asymmetry. Biometals 25:309–318. https://doi.org/10.1007/s10534-011-9507-5
Su TY, Pan CH, Hsu YT, Lai CH (2019) Effects of heavy metal exposure on shipyard welders: a cautionary note for 8-hydroxy-2’deoxyguanosine. Int J Environ Res Public Health 16:4813. https://doi.org/10.3390/ijerph16234813
Author information
Authors and Affiliations
Contributions
Mine Köktürk: conducting experiments and proofing manuscript, Serkan Yildirim: conducting experiments, Gizem Eser: conducting experiments, Menekşe Bulut: conducting experiments, Duried Alwazeer: supervisor and drafting the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate.
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Data Availability Statements
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
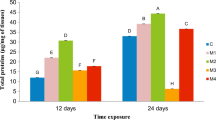
• Cocoon production of earthworms exposed to nickel was ameliorated in the presence of hydrogen-rich water
• Hydrogen-rich water attenuated nickel-induced oxidative stress in earthworms by decreasing ROS levels
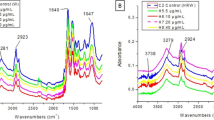
• Hydrogen-rich water reduced DNA damage (8-OHdG) caused by high nickel exposure
• Hydrogen-rich water reduced inflammatory responses (TNF-α) produced by nickel exposure
Rights and permissions
About this article
Cite this article
Köktürk, M., Yıldırım, S., Eser, G. et al. Hydrogen-Rich Water Alleviates the Nickel-Induced Toxic Responses (Inflammatory Responses, Oxidative Stress, DNA Damage) and Ameliorates Cocoon Production in Earthworm. Biol Trace Elem Res 200, 3442–3452 (2022). https://doi.org/10.1007/s12011-021-02908-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02908-7