Abstract
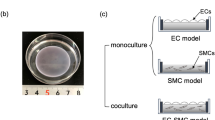
Though previous studies have indicated a relationship between the proliferation of endothelial cells and vascular smooth muscle cells (VSMCs) in co-culture, the results have been contradictory and the signaling mechanism poorly understood. In this transmembrane co-culture study, VSMCs and endothelial cells were grown to confluence on opposite sides of a microporous membrane to mimic the intima/media border of vessels. The endothelial layer was injured, and then cultured for 3 days, resulting in partial re-endothelialization. VSMC proliferation across from the injured/partially recovered endothelial region was significantly higher than across from the de-endothelialized region (a sevenfold increase) and the uninjured region (a threefold increase). ELISA indicated that PDGF, which was undetectable in uninjured co-culture and homotypic controls, increased after injury and the addition of a piperazinyl-quinazoline carboxamide PDGF receptor inhibitor blocked VSMC proliferation across from the injured/partially recovered region. We conclude that co-culture signaling initiated by endothelial cell injury locally stimulates VSMC proliferation and that this signaling could be mediated by PDGF-BB.






Similar content being viewed by others
References
Belkin, M., & Whittemore, A. D. (2000). Infrainguinal bypass. In R. B. Rutherford (Ed.), Vascular surgery (5th ed., pp. 998–1018). Philadelphia: W.B. Saunders Company.
Thyberg, J., Blomgren, K., Roy, J., Tran, P. K., & Hedin, U. (1997). Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. The Journal of Histochemistry and Cytochemistry, 45, 837–846.
Dilley, R. J., McGeachie, J. K., & Prendergast, F. J. (1987). A review of the proliferative behaviour, morphology and phenotypes of vascular smooth muscle. Atherosclerosis, 63, 99–107.
Painter, T. A. (1991). Myointimal hyperplasia: pathogenesis and implications. 2. Animal injury models and mechanical factors. Artificial Organs, 15, 103–118.
Conte, M. S. (1998). The ideal small arterial substitute: a search for the Holy Grail? The FASEB Journal, 12, 43–45.
Jacot, J. G., Abdullah, I., Belkin, M., Gerhard-Herman, M., Gaccione, P., Polak, J., et al. (2004). Early adaptation of human lower extremity vein grafts: Wall stiffness changes accompany geometric remodeling. Journal of Vascular Surgery, 39, 547–555.
Mann, M., Whittemore, A. D., Donaldson, M., Belkin, M., Conte, M. S., Polak, J., et al. (1999). Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet, 354, 1493–1498.
Smith, E. J., & Rothman, M. T. (2003). Antiproliferative coatings for the treatment of coronary heart disease: what are the targets and which are the tools? Journal of Interventional Cardiology, 16, 475–483.
Marx, M., Perlmutter, R. A., & Madri, J. A. (1994). Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. The Journal of Clinical Investigation, 93, 131–139.
Levitzki, A. (2005). PDGF receptor kinase inhibitors for the treatment of restenosis. Cardiovascular Research, 65, 581–586.
Fillinger, M. F., Sampson, L. N., Cronenwett, J. L., Powell, R. J., & Wagner, R. J. (1997). Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. The Journal of Surgical Research, 67, 169–178.
Axel, D. I., Brehm, B., Wolburg-Buchholz, K., Betz, E., Koveker, G., & Karsch, K. R. (1996). Induction of cell-rich and lipid-rich plaques in a transfilter coculture system with human vascular cells. Journal of Vascular Research, 33, 327–339.
Axel, D. I., Riessen, R., Athanasiadis, A., Runge, H., Koveker, G., & Karsch, K. R. (1997). Growth factor expression of human arterial smooth muscle cells and endothelial cells in a transfilter coculture system. Journal of Molecular and Cellular Cardiology, 29, 2967–2978.
Hastings, N. E., Simmers, M. B., McDonald, O. G., Wamhoff, B. R., & Blackman, B. R. (2007). Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. American Journal of Physiology Cell Physiology, 293, C1824–C1833.
Isakson, B. E., & Duling, B. R. (2005). Heterocellular contact at the myoendothelial junction influences gap junction organization. Circulation Research, 97, 44–51.
Matsuno, K., Ushiki, J., Seishi, T., Ichimura, M., Giese, N. A., Yu, J. C., et al. (2003). Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. 3. Replacement of quinazoline moiety and improvement of metabolic polymorphism of 4-[4-(N-substituted (thio)carbamoyl)-1-piperazinyl]-6,7-dimethoxyquinazoline derivatives. Journal of Medicinal Chemistry, 46, 4910–4925.
Lavado, E., Sanchez-Abarca, L. I., Tabernero, A., Bolanos, J. P., & Medina, J. M. (1997). Oleic acid inhibits gap junction permeability and increases glucose uptake in cultured rat astrocytes. Journal of Neurochemistry, 69, 721–728.
Powell, R. J., Cronenwell, J., Fillinger, M., & Wagner, R. (1994). Effect of endothelial cells and transforming growth factor-beta1 on cultured vascular smooth muscle cell growth patterns. Journal of Vascular Surgery, 20, 787–794.
Nugent, M., Karnovsky, M., & Edelman, E. (1993). Vascular cell-derived heparin sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circulation Research, 73, 1051–1060.
Cucina, A., Borrelli, V., Randone, B., Coluccia, P., Sapienza, P., & Cavallaro, A. (2003). Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. Journal of Surgical Research, 109, 16–23.
Rose, S. L., & Babensee, J. E. (2008). Smooth muscle cell phenotype alters cocultured endothelial cell response to biomaterial-pretreated leukocytes. Journal of Biomedical Materials Research Part A, 84, 661–671.
Chiu, J. J., Chen, L. J., Lee, C. I., Lee, P. L., Lee, D. Y., Tsai, M. C., et al. (2007). Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood, 110, 519–528.
Lavender, M. D., Pang, Z., Wallace, C. S., Niklason, L. E., & Truskey, G. A. (2005). A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials, 26, 4642–4653.
Leung, B. M., & Sefton, M. V. (2007). A modular tissue engineering construct containing smooth muscle cells and endothelial cells. Annals of Biomedical Engineering, 35, 2039–2049.
Chen, D., Walsh, K., & Wang, J. (2000). Regulation of cdk2 activity in endothelial cells that are inhibited from growth by cell contact. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 629–635.
Ettenson, D. S., & Gotlieb, A. I. (1994). Endothelial wounds with disruption in cell migration repair primarily by cell proliferation. Microvascular Research, 48, 328–337.
Farooqui, R., & Fenteany, G. (2005). Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. Journal of Cell Science, 118, 51–63.
Stavri, G. T., Zachary, I., Baskerville, P. A., Martin, J. F., & Erusalimsky, J. D. (1995). Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells: Synergistic interaction with hypoxia. Circulation, 92, 11–14.
Zachary, I. (2001). Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. American Journal of Physiology Cell Physiology, 280, C1375–C1386.
Tanner, F. C., Meier, P., Greutert, H., Champion, C., Nabel, E. G., & Luscher, T. F. (2000). Nitric oxide modulates expression of cell cycle regulatory proteins: A cytostatic strategy for inhibition of human vascular smooth muscle cell proliferation. Circulation, 101, 1982–1989.
Napoli, C., & Ignarro, L. J. (2001). Nitric oxide and atherosclerosis. Nitric Oxide, 5, 88–97.
Dora, K. A. (2001). Cell-cell communication in the vessel wall. Vascular Medicine, 6, 43–50.
Lindner, V., & Reidy, M. A. (1995). Platelet-derived growth factor ligand and receptor expression by large vessel endothelium in vivo. American Journal of Pathology, 146, 1488–1497.
Lindner, V., Giachelli, C. M., Schwartz, S. M., & Reidy, M. A. (1995). A subpopulation of smooth muscle cells in injured rat arteries expresses platelet-derived growth factor-B chain mRNA. Circulation Research, 76, 951–957.
Ferns, G. A., Raines, E. W., Sprugel, K. H., Motani, A. S., Reidy, M. A., & Ross, R. (1991). Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science, 253, 1129–1132.
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A., & Betsholtz, C. (1999). Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development, 126, 3047–3055.
Fredriksson, L., Li, H., & Eriksson, U. (2004). The PDGF family: four gene products form five dimeric isoforms. Cytokine & Growth Factor Reviews, 15, 197–204.
Little, T. L., Xia, J., & Duling, B. R. (1995). Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circulation Research, 76, 498–504.
Christ, G. J., Spray, D. C., el-Sabban, M., Moore, L. K., & Brink, P. R. (1996). Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circulation Research, 79, 631–646.
Deglise, S., Martin, D., Probst, H., Saucy, F., Hayoz, D., Waeber, G., et al. (2005). Increased connexin43 expression in human saphenous veins in culture is associated with intimal hyperplasia. Journal of Vascular Surgery, 41, 1043–1052.
Isakson, B. E., Ramos, S. I., & Duling, B. R. (2007). Ca2+ and inositol 1, 4, 5-trisphosphate-mediated signaling across the myoendothelial junction. Circulation Research, 100, 246–254.
Li, W. E., Waldo, K., Linask, K. L., Chen, T., Wessels, A., Parmacek, M. S., et al. (2002). An essential role for connexin43 gap junctions in mouse coronary artery development. Development, 129, 2031–2042.
Fisher, W. E., Boros, L. G., & Schirmer, W. J. (1995). Reversal of enhanced pancreatic cancer growth in diabetes by insulin. Surgery, 118, 453–457.
Bornfeldt, K. E., Raines, E. W., Graves, L. M., Skinner, M. P., Krebs, E. G., & Ross, R. (1995). Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Annals of the New York Academy of Sciences, 766, 416–430.
Nackman, G. B., Fillinger, M. F., Shafritz, R., Wei, T., & Graham, A. M. (1998). Flow modulates endothelial regulation of smooth muscle cell proliferation: A new model. Surgery, 124, 353–360. discussion 360–1.
Sakamoto, N., Ohashi, T., & Sato, M. (2006). Effect of fluid shear stress on migration of vascular smooth muscle cells in cocultured model. Annals of Biomedical Engineering, 34, 408–415.
Wang, H. Q., Huang, L. X., Qu, M. J., Yan, Z. Q., Liu, B., Shen, B. R., et al. (2006). Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium, 13, 171–180.
Redmond, E. M., Cullen, J. P., Cahill, P. A., Sitzmann, J. V., Stefansson, S., Lawrence, D. A., et al. (2001). Endothelial cells inhibit flow-induced smooth muscle cell migration: Role of plasminogen activator inhibitor-1. Circulation, 103, 597–603.
Palumbo, R., Gaetano, C., Antonini, A., Pompilio, G., Bracco, E., Ronnstrand, L., et al. (2002). Different effects of high and low shear stress on platelet-derived growth factor isoform release by endothelial cells: Consequences for smooth muscle cell migration. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 405–411.
Aromatario, C., Sterpetti, A. V., Palumbo, R., Patrizi, A. L., Di Carlo, A., Proietti, P., et al. (1997). Fluid shear stress increases the release of platelet derived growth factor BB (PDGF BB) by aortic endothelial cells. Minerva Cardioangiologica, 45, 1–7.
Dardik, A., Yamashita, A., Aziz, F., Asada, H., & Sumpio, B. E. (2005). Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1alpha. Journal of Vascular Surgery, 41, 321–331.
DePaola, N., Davies, P. F., Pritchard, W. F., Jr., Florez, L., Harbeck, N., & Polacek, D. C. (1999). Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proceedings of the National Academy of Sciences of the United States of America, 96, 3154–3159.
Inai, T., Mancuso, M. R., McDonald, D. M., Kobayashi, J., Nakamura, K., & Shibata, Y. (2004). Shear stress-induced upregulation of connexin 43 expression in endothelial cells on upstream surfaces of rat cardiac valves. Histochemistry and Cell Biology, 122, 477–483.
Cowan, D. B., Lye, S. J., & Langille, B. L. (1998). Regulation of vascular connexin43 gene expression by mechanical loads. Circulation Research, 82, 786–793.
Johnson, T. L., & Nerem, R. M. (2007). Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium, 14, 215–226.
Chiu, J. J., Chen, L. J., Lee, P. L., Lee, C. I., Lo, L. W., Usami, S., et al. (2003). Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood, 101, 2667–2674.
Chiu, J. J., Chen, L. J., Chen, C. N., Lee, P. L., & Lee, C. I. (2004). A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. Journal of Biomechanics, 37, 531–539.
Chiu, J. J., Chen, L. J., Chang, S. F., Lee, P. L., Lee, C. I., Tsai, M. C., et al. (2005). Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: Role of NF-kappaB. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 963–969.
Kristek, F., & Gerova, M. (1992). Myoendothelial relations in the conduit coronary artery of the dog and rabbit. Journal of Vascular Research, 29, 29–32.
Sosa-Melgarejo, J. A., & Berry, C. L. (1992). Myoendothelial contacts in the thoracic aorta of rat fetuses. Journal of Pathology, 166, 311–316.
Sosa-Melgarejo, J. A., & Berry, C. L. (1995). Myoendothelial contacts in the human fetal aorta. Archives of Medical Research, 26, 431–435.
Sosa-Melgarejo, J. A., Berry, C. L., & Dodd, S. (1988). Myoendothelial contacts in the small arterioles of human kidney. Virchows Archiv. A, Pathological Anatomy and Histopathology, 413, 183–187.
Sosa-Melgarejo, J. A., & Berry, C. L. (1992). Myoendothelial contacts in arteriolosclerosis. Journal of Pathology, 167, 235–239.
Haas, T. L., & Duling, B. R. (1997). Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvascular Research, 53, 113–120.
Sandow, S. L., & Hill, C. E. (2000). Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circulation Research, 86, 341–346.
Griffith, T. M., Chaytor, A. T., Bakker, L. M., & Edwards, D. H. (2005). 5-Methyltetrahydrofolate and tetrahydrobiopterin can modulate electrotonically mediated endothelium-dependent vascular relaxation. Proceedings of the National Academy of Sciences of the United States of America, 102, 7008–7013.
Martin, P. E., Wall, C., & Griffith, T. M. (2005). Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. British Journal of Pharmacology, 144, 617–627.
Acknowledgments
We thank P. Cook and M. Nugent for helpful discussions, E. Bartolak-Suki for critical reading of the manuscript, and C. Piron and E. Cosgrove for technical assistance with image analysis. This work was supported by the National Institute of Health (R01 HL72900-01), NASA (NAG9-1558) and a National Science Foundation CAREER Award (BES-9985338) to J.Y.W.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jacot, J.G., Wong, J.Y. Endothelial Injury Induces Vascular Smooth Muscle Cell Proliferation in Highly Localized Regions of a Direct Contact Co-culture System. Cell Biochem Biophys 52, 37–46 (2008). https://doi.org/10.1007/s12013-008-9023-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-008-9023-6