Abstract
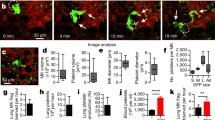
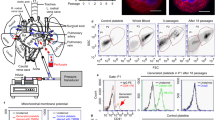
Platelets are released from megakaryocytes. The bone marrow has been proposed to be the major site where this process occurs. Lefrançais et al. (2017) using state-of-the-art techniques including two-photon microscopy, in vivo lineage-tracing technologies, and sophisticated lung transplants reveal that the lung is also a primary site for platelet biogenesis. Strikingly, lung megakaryocytes can completely reconstitute platelet counts in the blood in mice with thrombocytopenia. This study also shows that hematopoietic progenitors, with capacity to repopulate the bone marrow after irradiation, are present in the lungs. This work brings a novel unexpected role for the lung as a niche for hematopoiesis. The emerging knowledge from this research may be important for the treatment of several disorders.

Similar content being viewed by others
References
Machlus, K. R., Thon, J. N., & Italiano Jr., J. E. (2014). Interpreting the developmental dance of the megakaryocyte: A review of the cellular and molecular processes mediating platelet formation. British Journal of Haematology, 165(2), 227–236.
He, S., Ekman, G. J., & Hedner, U. (2005). The effect of platelets on fibrin gel structure formed in the presence of recombinant factor VIIa in hemophilia plasma and in plasma from a patient with Glanzmann thrombasthenia. Journal of Thrombosis and Haemostasis : JTH, 3(2), 272–279.
Ho-Tin-Noe, B., Demers, M., & Wagner, D. D. (2011). How platelets safeguard vascular integrity. Journal of Thrombosis and Haemostasis : JTH, 9(Suppl 1), 56–65.
Bates, E. R., & Lau, W. C. (2005). Controversies in antiplatelet therapy for patients with cardiovascular disease. Circulation, 111(17), e267–e271.
Laki, K. (1972). Our ancient heritage in blood clotting and some of its consequences. Annals of the New York Academy of Sciences, 202, 297–307.
Osman, A., Hitzler, W. E., & Provost, P. (2017). The platelets’ perspective to pathogen reduction technologies. Platelets, 1–8.
Weyrich, A. S., & Zimmerman, G. A. (2004). Platelets: Signaling cells in the immune continuum. Trends in Immunology, 25(9), 489–495.
Tesfamariam, B. (2016). Involvement of platelets in tumor cell metastasis. Pharmacology & Therapeutics, 157, 112–119.
Smyth, S. S., McEver, R. P., Weyrich, A. S., Morrell, C. N., Hoffman, M. R., Arepally, G. M., French, P. A., Dauerman, H. L., Becker, R. C., & Platelet Colloquium, P. (2009). Platelet functions beyond hemostasis. Journal of Thrombosis and Haemostasis : JTH, 7(11), 1759–1766.
Semple, J. W., Italiano Jr., J. E., & Freedman, J. (2011). Platelets and the immune continuum. Nature Reviews Immunology, 11(4), 264–274.
Davi, G., & Patrono, C. (2007). Platelet activation and atherothrombosis. The New England Journal of Medicine, 357(24), 2482–2494.
Engelmann, B., & Massberg, S. (2013). Thrombosis as an intravascular effector of innate immunity. Nature Reviews Immunology, 13(1), 34–45.
Danielli, J. F. (1940). Capillary permeability and oedema in the perfused frog. The Journal of Physiology, 98(1), 109–129.
Robb-Smith, A. H. (1967). Why the platelets were discovered. British Journal of Haematology, 13(4), 618–637.
Pease, D. C. (1956). An electron microscopic study of red bone marrow. Blood, 11(6), 501–526.
Italiano Jr., J. E., Lecine, P., Shivdasani, R. A., & Hartwig, J. H. (1999). Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. The Journal of Cell Biology, 147(6), 1299–1312.
Nakeff, A., & Maat, B. (1974). Separation of megakaryocytes from mouse bone marrow by velocity sedimentation. Blood, 43(4), 591–595.
Weyrich, A. S., & Zimmerman, G. A. (2013). Platelets in lung biology. Annual Review of Physiology, 75, 569–591.
Geddis, A. E., & Kaushansky, K. (2007). Immunology. The root of platelet production. Science, 317(5845), 1689–1691.
Howell, W. H., & Donahue, D. D. (1937). The production of blood platelets in the lungs. The Journal of Experimental Medicine, 65(2), 177–203.
Kallinikos-Maniatis, A. (1969). Megakaryocytes and platelets in central venous and arterial blood. Acta Haematologica, 42(6), 330–335.
Xiao da, W., Yang, M., Yang, J., Hon, K. L., & Fok, F. T. (2006). Lung damage may induce thrombocytopenia. Platelets, 17(5), 347–349.
Lefrancais, E., Ortiz-Munoz, G., Caudrillier, A., Mallavia, B., Liu, F., Sayah, D. M., Thornton, E. E., Headley, M. B., David, T., Coughlin, S. R., et al. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature, 544(7648), 105–109.
Sola-Visner, M. C., Christensen, R. D., Hutson, A. D., & Rimsza, L. M. (2007). Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatric Research, 61(4), 479–484.
Pang, L., Weiss, M. J., & Poncz, M. (2005). Megakaryocyte biology and related disorders. The Journal of Clinical Investigation, 115(12), 3332–3338.
Long, M. W., Williams, N., & Ebbe, S. (1982). Immature megakaryocytes in the mouse: Physical characteristics, cell cycle status, and in vitro responsiveness to thrombopoietic stimulatory factor. Blood, 59(3), 569–575.
Gordon, M. Y., Bearpark, A. D., Clarke, D., & Dowding, C. R. (1990). Haemopoietic stem cell subpopulations in mouse and man: Discrimination by differential adherence and marrow repopulating ability. Bone Marrow Transplantation, 5(Suppl 1), 6–8.
Ogawa, M. (1993). Differentiation and proliferation of hematopoietic stem cells. Blood, 81(11), 2844–2853.
Morita, Y., Iseki, A., Okamura, S., Suzuki, S., Nakauchi, H., & Ema, H. (2011). Functional characterization of hematopoietic stem cells in the spleen. Experimental Hematology, 39(3), 351–359 e353.
Gross, S., & Luckey, C. (1969). The oxygen tension-platelet relationship in cystic fibrosis. The American Review of Respiratory Disease, 100(4), 513–517.
O'Sullivan, B. P., & Michelson, A. D. (2006). The inflammatory role of platelets in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine, 173(5), 483–490.
Kemona-Chetnik, I., Bodzenta-Lukaszyk, A., Butkiewicz, A., & Dymnicka-Piekarska, V. (2007). Kemona H: [Thrombocytopoesis in allergic asthma]. Polskie Archiwum Medycyny Wewnętrznej, 117(1–2), 9–13.
Kornerup, K. N., & Page, C. P. (2007). The role of platelets in the pathophysiology of asthma. Platelets, 18(5), 319–328.
Stoll, P., & Lommatzsch, M. (2014). Platelets in asthma: Does size matter? Respiration; International Review of Thoracic Diseases, 88(1), 22–23.
Tozkoparan, E., Deniz, O., Ucar, E., Bilgic, H., & Ekiz, K. (2007). Changes in platelet count and indices in pulmonary tuberculosis. Clinical Chemistry and Laboratory Medicine, 45(8), 1009–1013.
Gunluoglu, G., Yazar, E. E., Veske, N. S., Seyhan, E. C., & Altin, S. (2014). Mean platelet volume as an inflammation marker in active pulmonary tuberculosis. Multidisciplinary Respiratory Medicine, 9(1), 11.
Kroll, M. H., & Afshar-Kharghan, V. (2012). Platelets in pulmonary vascular physiology and pathology. Pulmonary Circulation, 2(3), 291–308.
Al-Drees, M. A., Yeo, J. H., Boumelhem, B. B., Antas, V. I., Brigden, K. W., Colonne, C. K., & Fraser, S. T. (2015). Making blood: The Haematopoietic niche throughout ontogeny. Stem Cells International, 2015, 571893.
Palis, J., Robertson, S., Kennedy, M., Wall, C., & Keller, G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development, 126(22), 5073–5084.
Tavian, M., & Peault, B. (2005). Embryonic development of the human hematopoietic system. The International Journal of Developmental Biology, 49(2–3), 243–250.
Bowman, T. V., & Zon, L. I. (2009). Lessons from the niche for generation and expansion of hematopoietic stem cells. Drug Discovery Today Therapeutic Strategies, 6(4), 135–140.
Swain, A., Inoue, T., Tan, K. S., Nakanishi, Y., & Sugiyama, D. (2014). Intrinsic and extrinsic regulation of mammalian hematopoiesis in the fetal liver. Histology and Histopathology, 29(9), 1077–1082.
Tanaka, Y., Inoue-Yokoo, T., Kulkeaw, K., Yanagi-Mizuochi, C., Shirasawa, S., Nakanishi, Y., & Sugiyama, D. (2015). Embryonic hematopoietic progenitor cells reside in muscle before bone marrow hematopoiesis. PloS One, 10(9), e0138621.
Medvinsky, A. L., Samoylina, N. L., Muller, A. M., & Dzierzak, E. A. (1993). An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature, 364(6432), 64–67.
Medvinsky, A., Rybtsov, S., & Taoudi, S. (2011). Embryonic origin of the adult hematopoietic system: Advances and questions. Development, 138(6), 1017–1031.
Baron, M. H. (2005). Early patterning of the mouse embryo: Implications for hematopoietic commitment and differentiation. Experimental Hematology, 33(9), 1015–1020.
Baron, M. H., Isern, J., & Fraser, S. T. (2012). The embryonic origins of erythropoiesis in mammals. Blood, 119(21), 4828–4837.
Barminko, J., Reinholt, B., & Baron, M. H. (2016). Development and differentiation of the erythroid lineage in mammals. Developmental and Comparative Immunology, 58, 18–29.
Kumaravelu, P., Hook, L., Morrison, A. M., Ure, J., Zhao, S., Zuyev, S., Ansell, J., & Medvinsky, A. (2002). Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): Role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development, 129(21), 4891–4899.
Muller, A. M., Medvinsky, A., Strouboulis, J., Grosveld, F., & Dzierzak, E. (1994). Development of hematopoietic stem cell activity in the mouse embryo. Immunity, 1(4), 291–301.
Medvinsky, A., & Dzierzak, E. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell, 86(6), 897–906.
Sugiyama, D., & Tsuji, K. (2006). Definitive hematopoiesis from endothelial cells in the mouse embryo; a simple guide. Trends in Cardiovascular Medicine, 16(2), 45–49.
Lux, C. T., Yoshimoto, M., McGrath, K., Conway, S. J., Palis, J., & Yoder, M. C. (2008). All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood, 111(7), 3435–3438.
Khan, J. A., Mendelson, A., Kunisaki, Y., Birbrair, A., Kou, Y., Arnal-Estape, A., Pinho, S., Ciero, P., Nakahara, F., Ma'ayan, A., et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science, 351(6269), 176–180.
Bozzini, C. E., Barrio Rendo, M. E., Devoto, F. C., & Epper, C. E. (1970). Studies on medullary and extramedullary erythropoiesis in the adult mouse. The American Journal of Physiology, 219(3), 724–728.
Bowen, J. M., Perry, A. M., Quist, E., & Akhtari, M. (2015). Extramedullary hematopoiesis in a sentinel lymph node as an early sign of chronic myelomonocytic leukemia. Case Reports in Pathology, 2015, 594970.
Schnuelle, P., Waldherr, R., Lehmann, K. J., Woenckhaus, J., Back, W., Niemir, Z., & van der Woude, F. J. (1999). Idiopathic myelofibrosis with extramedullary hematopoiesis in the kidneys. Clinical Nephrology, 52(4), 256–262.
Woodward, N., Ancliffe, P., Griffiths, M. H., & Cohen, S. (2000). Renal myelofibrosis: An unusual cause of renal impairment. Nephrology, Dialysis, Transplantation, 15(2), 257–258.
Lewis, D. J., Moul, J. W., Williams, S. C., Sesterhenn, I. A., & Colon, E. (1994). Perirenal liposarcoma containing extramedullary hematopoiesis associated with renal cell carcinoma. Urology, 43(1), 106–109.
Guenechea, G., Gan, O. I., Dorrell, C., & Dick, J. E. (2001). Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nature Immunology, 2(1), 75–82.
Ema, H., Sudo, K., Seita, J., Matsubara, A., Morita, Y., Osawa, M., Takatsu, K., Takaki, S., & Nakauchi, H. (2005). Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Developmental Cell, 8(6), 907–914.
Yamamoto, R., Morita, Y., Ooehara, J., Hamanaka, S., Onodera, M., Rudolph, K. L., Ema, H., & Nakauchi, H. (2013). Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell, 154(5), 1112–1126.
Purton, L. E., & Scadden, D. T. (2007). Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell, 1(3), 263–270.
Kunisaki, Y., Bruns, I., Scheiermann, C., Ahmed, J., Pinho, S., Zhang, D., Mizoguchi, T., Wei, Q., Lucas, D., Ito, K., et al. (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature, 502(7473), 637–643.
Asada N., Kunisaki Y., Pierce H., Wang Z., Fernandez N.F., Birbrair A., Ma’ayan A., Frenette P. S. (2017). Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nature cell biology.
Schofield, R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells, 4(1–2), 7–25.
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C., & Morrison, S. J. (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell, 121(7), 1109–1121.
Sena, I., Prazeres, P., Santos, G., Borges, I., Azevedo, P., Andreotti, J., Almeida, V., Paiva, A., Guerra, D., Lousado, L., Souto, L., Mintz, A., & Birbrair, A (2017). Identity of Gli1+ cells in the bone marrow. Experimental Hematology. In press
Birbrair, A., & Delbono, O. (2015). Pericytes are essential for skeletal muscle formation. Stem Cell Reviews, 11(4), 547–548.
Birbrair A., Frenette P.S. (2016). Niche heterogeneity in the bone marrow. Annals of the new York Academy of Sciences, 1370, 82–96.
Birbrair, A., Zhang, T., Files, D. C., Mannava, S., Smith, T., Wang, Z. M., Messi, M. L., Mintz, A., & Delbono, O. (2014). Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Research & Therapy, 5(6), 122.
Birbrair, A., Zhang, T., Wang, Z. M., Messi, M. L., Enikolopov, G. N., Mintz, A., & Delbono, O. (2013). Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Research, 10(1), 67–84.
Birbrair, A., Zhang, T., Wang, Z. M., Messi, M. L., Enikolopov, G. N., Mintz, A., & Delbono, O. (2013). Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells and Development, 22(16), 2298–2314.
Birbrair, A., Zhang, T., Wang, Z. M., Messi, M. L., Mintz, A., & Delbono, O. (2013). Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. American Journal of Physiology. Cell Physiology, 305(11), C1098–C1113.
Birbrair, A., Zhang, T., Wang, Z. M., Messi, M. L., Mintz, A., & Delbono, O. (2014). Pericytes: Multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Frontiers in Aging Neuroscience, 6, 245.
Birbrair, A., Zhang, T., Wang, Z. M., Messi, M. L., Mintz, A., & Delbono, O. (2015). Pericytes at the intersection between tissue regeneration and pathology. Clinical Science (London, England), 128(2), 81–93.
Birbrair, A., Zhang, T., Wang, Z. M., Messi, M. L., Olson, J. D., Mintz, A., & Delbono, O. (2014). Type-2 pericytes participate in normal and tumoral angiogenesis. American Journal of Physiology. Cell Physiology, 307(1), C25–C38.
Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro dos Santos GS, Mintz A. et al. (2017) Pericytes are heterogeneous in their origin within the same tissue. Developmental Biology.
Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O (2017) How plastic are pericytes? Stem cells and development.
Birbrair, A., Zhang T., Wang ZM., Messi ML., Enikolopov, GN., Mintz, A., & Delbono, O. (2013). Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Experimental Cell Research, 319(1), 45-63.
Birbrair, A., Wang, ZM., Messi, ML., Enikolopov, GN., Delbono, O., & Rota, M. (2011) Nestin-GFP Transgene Reveals Neural Precursor Cells in Adult Skeletal Muscle. PLoS ONE, 6(2), e16816.
Birbrair, A., Sattiraju, A., Zhu, D., Zulato, G., Batista, B., Nguyen, VT. et al. (2017) Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas. STEM CELLS Translational Medicine, 6(2), 471–481.
Rhodes, K. E., Gekas, C., Wang, Y., Lux, C. T., Francis, C. S., Chan, D. N., Conway, S., Orkin, S. H., Yoder, M. C., & Mikkola, H. K. (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell, 2(3), 252–263.
Crapo, J. D., Barry, B. E., Gehr, P., Bachofen, M., & Weibel, E. R. (1982). Cell number and cell characteristics of the normal human lung. The American Review of Respiratory Disease, 126(2), 332–337.
Bruns, I., Lucas, D., Pinho, S., Ahmed, J., Lambert, M. P., Kunisaki, Y., Scheiermann, C., Schiff, L., Poncz, M., Bergman, A., et al. (2014). Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nature Medicine, 20(11), 1315–1320.
Nakamura-Ishizu, A., Takubo, K., Fujioka, M., & Suda, T. (2014). Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochemical and Biophysical Research Communications, 454(2), 353–357.
Heazlewood, S. Y., Neaves, R. J., Williams, B., Haylock, D. N., Adams, T. E., & Nilsson, S. K. (2013). Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Research, 11(2), 782–792.
Kaushansky, K., Lok, S., Holly, R. D., Broudy, V. C., Lin, N., Bailey, M. C., Forstrom, J. W., Buddle, M. M., Oort, P. J., Hagen, F. S., et al. (1994). Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature, 369(6481), 568–571.
Kimura, S., Roberts, A. W., Metcalf, D., & Alexander, W. S. (1998). Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 1195–1200.
Bersenev, A., Wu, C., Balcerek, J., & Tong, W. (2008). Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. The Journal of Clinical Investigation, 118(8), 2832–2844.
Yoshihara, H., Arai, F., Hosokawa, K., Hagiwara, T., Takubo, K., Nakamura, Y., Gomei, Y., Iwasaki, H., Matsuoka, S., Miyamoto, K., et al. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell, 1(6), 685–697.
de Graaf, C. A., Kauppi, M., Baldwin, T., Hyland, C. D., Metcalf, D., Willson, T. A., Carpinelli, M. R., Smyth, G. K., Alexander, W. S., & Hilton, D. J. (2010). Regulation of hematopoietic stem cells by their mature progeny. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21689–21694.
Zhao, M., Perry, J. M., Marshall, H., Venkatraman, A., Qian, P., He, X. C., Ahamed, J., & Li, L. (2014). Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nature Medicine, 20(11), 1321–1326.
Olson, T. S., Caselli, A., Otsuru, S., Hofmann, T. J., Williams, R., Paolucci, P., Dominici, M., & Horwitz, E. M. (2013). Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood, 121(26), 5238–5249.
Soderberg, S. S., Karlsson, G., & Karlsson, S. (2009). Complex and context dependent regulation of hematopoiesis by TGF-beta superfamily signaling. Annals of the New York Academy of Sciences, 1176, 55–69.
Kent, D. G., Copley, M. R., Benz, C., Wohrer, S., Dykstra, B. J., Ma, E., Cheyne, J., Zhao, Y., Bowie, M. B., Zhao, Y., et al. (2009). Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood, 113(25), 6342–6350.
Sanjuan-Pla, A., Macaulay, I. C., Jensen, C. T., Woll, P. S., Luis, T. C., Mead, A., Moore, S., Carella, C., Matsuoka, S., Bouriez Jones, T., et al. (2013). Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature, 502(7470), 232–236.
Weibel, E. R. (1974). On pericytes, particularly their existence on lung capillaries. Microvascular Research, 8(2), 218–235.
Hung, C., Linn, G., Chow, Y. H., Kobayashi, A., Mittelsteadt, K., Altemeier, W. A., Gharib, S. A., Schnapp, L. M., & Duffield, J. S. (2013). Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 188(7), 820–830.
Rock, J. R., Barkauskas, C. E., Cronce, M. J., Xue, Y., Harris, J. R., Liang, J., Noble, P. W., & Hogan, B. L. (2011). Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proceedings of the National Academy of Sciences of the United States of America, 108(52), E1475–E1483.
Ricard, N., Tu, L., Le Hiress, M., Huertas, A., Phan, C., Thuillet, R., Sattler, C., Fadel, E., Seferian, A., Montani, D., et al. (2014). Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation, 129(15), 1586–1597.
Shepro, D., & Morel, N. M. (1993). Pericyte physiology. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 7(11), 1031–1038.
Folman, C. C., Linthorst, G. E., van Mourik, J., van Willigen, G., de Jonge, E., Levi, M., de Haas, M., & von dem Borne, A. E. (2000). Platelets release thrombopoietin (Tpo) upon activation: Another regulatory loop in thrombocytopoiesis? Thrombosis and Haemostasis, 83(6), 923–930.
Levesque, J. P., Hendy, J., Winkler, I. G., Takamatsu, Y., & Simmons, P. J. (2003). Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Experimental Hematology, 31(2), 109–117.
Heissig, B., Hattori, K., Dias, S., Friedrich, M., Ferris, B., Hackett, N. R., Crystal, R. G., Besmer, P., Lyden, D., Moore, M. A., et al. (2002). Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell, 109(5), 625–637.
Petit, I., Szyper-Kravitz, M., Nagler, A., Lahav, M., Peled, A., Habler, L., Ponomaryov, T., Taichman, R. S., Arenzana-Seisdedos, F., Fujii, N., et al. (2002). G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology, 3(7), 687–694.
Valenzuela-Fernandez, A., Planchenault, T., Baleux, F., Staropoli, I., Le-Barillec, K., Leduc, D., Delaunay, T., Lazarini, F., Virelizier, J. L., Chignard, M., et al. (2002). Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. The Journal of Biological Chemistry, 277(18), 15677–15689.
Levesque, J. P., Takamatsu, Y., Nilsson, S. K., Haylock, D. N., & Simmons, P. J. (2001). Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood, 98(5), 1289–1297.
Chow, A., Huggins, M., Ahmed, J., Hashimoto, D., Lucas, D., Kunisaki, Y., Pinho, S., Leboeuf, M., Noizat, C., van Rooijen, N., et al. (2013). CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature Medicine, 19(4), 429–436.
Meyer, A., Wang, W., Qu, J., Croft, L., Degen, J. L., Coller, B. S., & Ahamed, J. (2012). Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood, 119(4), 1064–1074.
Labelle, M., Begum, S., & Hynes, R. O. (2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell, 20(5), 576–590.
Pinho, S., Lacombe, J., Hanoun, M., Mizoguchi, T., Bruns, I., Kunisaki, Y., & Frenette, P. S. (2013). PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of Experimental Medicine, 210(7), 1351–1367.
Notta, F., Doulatov, S., Laurenti, E., Poeppl, A., Jurisica, I., & Dick, J. E. (2011). Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science, 333(6039), 218–221.
Guezguez, B., Campbell, C. J., Boyd, A. L., Karanu, F., Casado, F. L., Di Cresce, C., Collins, T. J., Shapovalova, Z., Xenocostas, A., & Bhatia, M. (2013). Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell, 13(2), 175–189.
Lefrancais E., Ortiz-Munoz G., Caudrillier A., Mallavia B., Liu F., Sayah D.M., Thornton E.E., Headley M.B., David T., Coughlin S.R. et al. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature, 544(7648), 105–109.
Acknowledgements
Alexander Birbrair is supported by a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD). We thank Rosa Maria Esteves Arantes for her useful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors indicate no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Borges, I., Sena, I., Azevedo, P. et al. Lung as a Niche for Hematopoietic Progenitors. Stem Cell Rev and Rep 13, 567–574 (2017). https://doi.org/10.1007/s12015-017-9747-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9747-z