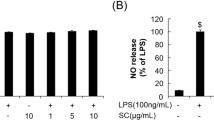
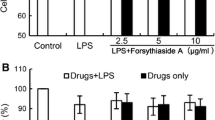
Abstract
Withania somnifera (L.) Dunal, commonly known as Ashwagandha, has been used in Ayurvedic medicine for promoting health and quality of life. Recent clinical trials together with experimental studies indicated significant neuroprotective effects of Ashwagandha and its constituents. This study is aimed to investigate anti-inflammatory and anti-oxidative properties of this botanical and its two withanolide constituents, namely, Withaferin A and Withanolide A, using the murine immortalized BV-2 microglial cells. Ashwagandha extracts not only effectively inhibited lipopolysaccharide (LPS)-induced nitric oxide (NO) and reactive oxygen species (ROS) production in BV-2 cells, but also stimulates the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway, leading to induction of heme oxygenase-1 (HO-1), both in the presence and absence of LPS. Although the withanolides were also capable of inhibiting LPS-induced NO production and stimulating Nrf2/HO-1 pathway, Withaferin A was tenfold more effective than Withanolide A. In serum-free culture, LPS can also induce production of long thin processes (filopodia) between 4 and 8 h in BV-2 cells. This morphological change was significantly suppressed by Ashwagandha and both withanolides at concentrations for suppressing LPS-induced NO production. Taken together, these results suggest an immunomodulatory role for Ashwagandha and its withanolides, and their ability to suppress oxidative and inflammatory responses in microglial cells by simultaneously down-regulating the NF-kB and upregulating the Nrf2 pathways.








Similar content being viewed by others
References
Ashkenazi, S., Plotnikov, A., Bahat, A., Ben-Zeev, E., Warszawski, S., & Dikstein, R. (2016). A novel allosteric mechanism of NF-κB dimerization and DNA binding targeted by an anti-inflammatory drug. Molecular and Cellular Biology, 36(8), 1237–1247.
Brandenburg, L. O., Kipp, M., Lucius, R., Pufe, T., & Wruck, C. J. (2010). Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflammation Research, 59(6), 443–450.
Calabrese, V., Cornelius, C., Dinkova-Kostova, A. T., Calabrese, E. J., & Mattson, M. P. (2010). Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxidants and Redox Signaling, 13(11), 1763–1811.
Chen, Z., & Trapp, B. D. (2016). Microglia and neuroprotection. Journal of Neurochemistry, 136(Suppl 1), 10–17.
Chen, J. C., Ho, F. M., Pei-Dawn Lee, C., Chen, C. P., Jeng, K. C., Hsu, H. B., et al. (2005). Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. European Journal of Pharmacology, 521(1–3), 9–20.
Chuang, D. Y., Chan, M. H., Zong, Y., Sheng, W., He, Y., Jiang, J. H., et al. (2013). Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. Journal of Neuroinflammation, 10, 15.
Chuang, D. Y., Simonyi, A., Kotzbauer, P. T., Gu, Z., & Sun, G. Y. (2015). Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. Journal of Neuroinflammation, 12, 199.
Dar, N. J., Hamid, A., & Ahmad, M. (2015). Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cellular and Molecular Life Sciences, 72(23), 4445–4460.
Durg, S., Dhadde, S. B., Vandal, R., Shivakumar, B. S., & Charan, C. S. (2015). Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: A systematic review and meta-analysis. Journal of Pharmacy and Pharmacology, 67(7), 879–899.
Foresti, R., Bains, S. K., Pitchumony, T. S., de Castro Bras, L. E., Drago, F., Dubois-Rande, J. L., et al. (2013). Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacological Research, 76, 132–148.
Gan, L., & Johnson, J. A. (2014). Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochimica et Biophysica Acta, 1842(8), 1208–1218.
Gao, B., Doan, A., & Hybertson, B. M. (2014). The clinical potential of influencing Nrf2 signaling in degenerative and immunological disorders. Clin Pharmacol, 6, 19–34.
Grin, B., Mahammad, S., Wedig, T., Cleland, M. M., Tsai, L., Herrmann, H., et al. (2012). Withaferin a alters intermediate filament organization, cell shape and behavior. PLoS One, 7(6), e39065.
Grunz-Borgmann, E., Mossine, V., Fritsche, K., & Parrish, A. R. (2015). Ashwagandha attenuates TNF-α- and LPS-induced NF-κB activation and CCL2 and CCL5 gene expression in NRK-52E cells. BMC Complementary and Alternative Medicine, 15, 434.
Innamorato, N. G., Rojo, A. I., Garcia-Yague, A. J., Yamamoto, M., de Ceballos, M. L., & Cuadrado, A. (2008). The transcription factor Nrf2 is a therapeutic target against brain inflammation. Journal of Immunology, 181(1), 680–689.
Jazwa, A., & Cuadrado, A. (2010). Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Current Drug Targets, 11(12), 1517–1531.
Jiang, J., Chuang, D. Y., Zong, Y., Patel, J., Brownstein, K., Lei, W., et al. (2014). Sutherlandia frutescens ethanol extracts inhibit oxidative stress and inflammatory responses in neurons and microglial cells. PLoS One, 9(2), e89748.
Joshi, G., & Johnson, J. A. (2012). The Nrf2-ARE pathway: A valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Patents on CNS Drug Discovery, 7(3), 218–229.
Kang, C. H., Choi, Y. H., Moon, S. K., Kim, W. J., & Kim, G. Y. (2013). Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. International Immunopharmacology, 17(3), 808–813.
Kobayashi, A., Kang, M. I., Okawa, H., Ohtsuji, M., Zenke, Y., Chiba, T., et al. (2004). Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and Cellular Biology, 24(16), 7130–7139.
Kuboyama, T., Tohda, C., & Komatsu, K. (2014). Effects of Ashwagandha (roots of Withania somnifera) on neurodegenerative diseases. Biological and Pharmaceutical Bulletin, 37(6), 892–897.
Kulkarni, S. K., & Dhir, A. (2008). Withania somnifera: An Indian ginseng. Progress in Neuropsychopharmacology and Biological Psychiatry, 32(5), 1093–1105.
Kurapati, K. R., Atluri, V. S., Samikkannu, T., & Nair, M. P. (2013). Ashwagandha (Withania somnifera) reverses beta-amyloid1-42 induced toxicity in human neuronal cells: implications in HIV-associated neurocognitive disorders (HAND). PLoS One, 8(10), e77624.
Kurapati, K. R., Atluri, V. S., Samikkannu, T., Garcia, G., & Nair, M. P. (2015). Natural products as anti-HIV agents and role in HIV-associated neurocognitive disorders (HAND): A brief overview. Frontiers in Microbiology, 6, 1444.
Lee, J., Jo, D. G., Park, D., Chung, H. Y., & Mattson, M. P. (2014). Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: Focus on the nervous system. Pharmacological Reviews, 66(3), 815–868.
Min, K. J., Choi, K., & Kwon, T. K. (2011). Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglial cells. International Immunopharmacology, 11(8), 1137–1142.
Mirjalili, M. H., Moyano, E., Bonfill, M., Cusido, R. M., & Palazon, J. (2009). Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules, 14(7), 2373–2393.
Nair, S., Doh, S. T., Chan, J. Y., Kong, A. N., & Cai, L. (2008). Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. British Journal of Cancer, 99(12), 2070–2082.
Narayan, M., Seeley, K. W., & Jinwal, U. K. (2015). Identification and quantitative analysis of cellular proteins affected by treatment with withaferin a using a SILAC-based proteomics approach. Journal of Ethnopharmacology, 175, 86–92.
Oh, J. H., Lee, T. J., Park, J. W., & Kwon, T. K. (2008). Withaferin A inhibits iNOS expression and nitric oxide production by Akt inactivation and down-regulating LPS-induced activity of NF-κB in RAW 264.7 cells. European Journal of Pharmacology, 599(1–3), 11–17.
Paine, A., Eiz-Vesper, B., Blasczyk, R., & Immenschuh, S. (2010). Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology, 80(12), 1895–1903.
Parada, E., Buendia, I., Navarro, E., Avendano, C., Egea, J., & Lopez, M. G. (2015). Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Molecular Nutrition and Food Research, 59(9), 1690–1700.
Patil, D., Gautam, M., Mishra, S., Karupothula, S., Gairola, S., Jadhav, S., et al. (2013). Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: Application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. Journal of Pharmaceutical and Biomedical Analysis, 80, 203–212.
Queiroga, C. S., Vercelli, A., & Vieira, H. L. (2015). Carbon monoxide and the CNS: Challenges and achievements. British Journal of Pharmacology, 172(6), 1533–1545.
Salter, M. W., & Beggs, S. (2014). Sublime microglia: Expanding roles for the guardians of the CNS. Cell, 158(1), 15–24.
Sandberg, M., Patil, J., D’Angelo, B., Weber, S. G., & Mallard, C. (2014). NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology, 79, 298–306.
Scapagnini, G., Vasto, S., Abraham, N. G., Caruso, C., Zella, D., & Fabio, G. (2011). Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Molecular Neurobiology, 44(2), 192–201.
Shah, N., Singh, R., Sarangi, U., Saxena, N., Chaudhary, A., Kaur, G., et al. (2015). Combinations of Ashwagandha leaf extracts protect brain-derived cells against oxidative stress and induce differentiation. PLoS One, 10(3), e0120554.
Sheng, W., Zong, Y., Mohammad, A., Ajit, D., Cui, J., Han, D., et al. (2011). Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA(2)-IIA expression in astrocytes and microglia. Journal of Neuroinflammation, 8, 121.
Simonyi, A., Chen, Z., Jiang, J., Zong, Y., Chuang, D. Y., Gu, Z., et al. (2015). Inhibition of microglial activation by elderberry extracts and its phenolic components. Life Sciences, 128, 30–38.
Singh, N., Bhalla, M., de Jager, P., & Gilca, M. (2011). An overview on ashwagandha: A Rasayana (rejuvenator) of Ayurveda. African Journal of Traditional, Complementary and Alternative Medicines, 8(5 Suppl), 208–213.
Soares, M. P., Marguti, I., Cunha, A., & Larsen, R. (2009). Immunoregulatory effects of HO-1: How does it work? Current Opinion in Pharmacology, 9(4), 482–489.
Sun, A. Y., Wang, Q., Simonyi, A., & Sun, G. Y. (2008). Botanical phenolics and brain health. Neuromolecular Medicine, 10(4), 259–274.
Sun, G. Y., Chen, Z., Jasmer, K. J., Chuang, D. Y., Gu, Z., Hannink, M., et al. (2015). Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS One, 10(10), e0141509.
Syapin, P. J. (2008). Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. British Journal of Pharmacology, 155(5), 623–640.
Terazawa, R., Akimoto, N., Kato, T., Itoh, T., Fujita, Y., Hamada, N., et al. (2013). A kavalactone derivative inhibits lipopolysaccharide-stimulated iNOS induction and NO production through activation of Nrf2 signaling in BV2 microglial cells. Pharmacological Research, 71, 34–43.
Vanden Berghe, W., Sabbe, L., Kaileh, M., Haegeman, G., & Heyninck, K. (2012). Molecular insight in the multifunctional activities of Withaferin A. Biochemical Pharmacology, 84(10), 1282–1291.
Ven Murthy, M. R., Ranjekar, P. K., Ramassamy, C., & Deshpande, M. (2010). Scientific basis for the use of Indian ayurvedic medicinal plants in the treatment of neurodegenerative disorders: Ashwagandha. Central Nervous System Agents in Medicinal Chemistry, 10(3), 238–246.
Vyas, A. R., & Singh, S. V. (2014). Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone. AAPS Journal, 16(1), 1–10.
Wadhwa, R., Konar, A., & Kaul, S. C. (2016). Nootropic potential of ashwagandha leaves: Beyond traditional root extracts. Neurochemistry International, 95, 109–118.
Wardyn, J. D., Ponsford, A. H., & Sanderson, C. M. (2015). Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochemical Society Transactions, 43(4), 621–626.
Zhang, D. D., Lo, S. C., Cross, J. V., Templeton, D. J., & Hannink, M. (2004). Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Molecular and Cellular Biology, 24(24), 10941–10953.
Acknowledgments
This work was partially supported by grant P50AT006273 from the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest for this study.
Rights and permissions
About this article
Cite this article
Sun, G.Y., Li, R., Cui, J. et al. Withania somnifera and Its Withanolides Attenuate Oxidative and Inflammatory Responses and Up-Regulate Antioxidant Responses in BV-2 Microglial Cells. Neuromol Med 18, 241–252 (2016). https://doi.org/10.1007/s12017-016-8411-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8411-0