Abstract
Background
Current guidelines recommend routine clamping of external ventricular drains (EVD) for intrahospital transport (IHT). The aim of this project was to describe intracranial hemodynamic complications associated with routine EVD clamping for IHT in neurocritically ill cerebrovascular patients.
Methods
We conducted a retrospective review of cerebrovascular adult patients with indwelling EVD admitted to the neurocritical care unit (NICU) during the months of September to December 2015 at a tertiary care center. All IHTs from the NICU of the included patients were examined. Main outcomes were incidence and risk factors for an alteration in intracranial pressure (ICP) and cerebral perfusion pressure after IHT.
Results
Nineteen cerebrovascular patients underwent 178 IHTs (79.8 % diagnostic and 20.2 % therapeutic) with clamped EVD. Twenty-one IHTs (11.8 %) were associated with post-IHT ICP ≥ 20 mmHg, and 33 IHTs (18.5 %) were associated with escalation of ICP category. Forty IHTs (26.7 %) in patients with open EVD status in the NICU prior to IHT were associated with IHT complications, whereas no IHT complications occurred in IHTs with clamped EVD status in the NICU. Risk factors for post-IHT ICP ≥ 20 mmHg were IHT for therapeutic procedures (adjusted relative risk [aRR] 5.82; 95 % CI, 1.76–19.19), pre-IHT ICP 15–19 mmHg (aRR 3.40; 95 % CI, 1.08–10.76), pre-IHT ICP ≥ 20 mmHg (aRR 12.94; 95 % CI, 4.08–41.01), and each 1 mL of hourly cerebrospinal fluid (CSF) drained prior to IHT (aRR 1.11; 95 % CI, 1.01–1.23).
Conclusions
Routine clamping of EVD for IHT in cerebrovascular patients is associated with post-IHT ICP complications. Pre-IHT ICP ≥ 15 mmHg, increasing hourly CSF output, and IHT for therapeutic procedures are risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahospital transport (IHT) of critically ill patients can be associated with perturbations in hemodynamic and respiratory parameters [1–5]. Neurocritically ill patients undergo IHT for computed tomography (CT), magnetic resonance imaging (MRI), and for procedures in the operating room and neuroradiology suites. In this group, secondary brain insults such as hypoxia, hypocarbia or hypercarbia, systemic hypotension, as well as high intracranial pressure (ICP) occurring during IHT can be position related or device related and may lead to reduction in cerebral perfusion pressure (CPP) and cerebral ischemia [2, 6–9].
An external ventricular drain (EVD) is the gold standard method when both ICP monitoring and external cerebrospinal fluid (CSF) diversion are required. Between 1988 and 2010, an estimated 500,000 ventriculostomy procedures were performed in the USA, most commonly in patients with subarachnoid hemorrhage (SAH) and intracerebral hemorrhage, with an increasing trend over time [10]. Current guidelines recommend routine clamping of EVD during IHT due to concerns for CSF overdrainage [11–13]. While typical hourly CSF drainage in adults is generally under 20 mL, complications of overdrainage at any time and over many days are not benign. Overdrainage of CSF from an EVD can certainly lead to intracranial hypotension, which in patients with a ruptured and unsecured cerebral aneurysm can promote re-bleed due to sudden increase in transmural pressure (MAP-ICP) [14–16]; subject patients to subdural hemorrhage due to bleeding from disruption of bridging veins [17–19]; and also potentially contribute to reverse brain herniation [20]. Intracranial hypertension has also been reported in many clinical studies on IHT in neurocritically ill patients, but none address specific EVD management during IHT [7, 9, 21]. A recently published prospective study by Kleffmann et al. [22] demonstrated ICP elevation during IHT in neurocritically ill patients. However, there was no discussion on EVD management in 50 % of the patients who had indwelling EVD.
Despite the guidelines and checklists that have been developed to improve the safety of critically ill patients during IHT [23–30], the frequency and severity of intracranial hemodynamic complications associated with routine EVD clamping are largely unknown. However, there is a lack of specific recommendations for testing tolerance to EVD clamping prior to initiation of IHT, regarding EVD clamping protocols or regarding ICP monitoring during IHT in neurocritically ill patients. Additionally, the most recently published guidelines for the management of EVD do not include IHT recommendations [31]. To bridge this gap in knowledge, we examined the incidence and factors associated with intracranial hemodynamic complications associated with routine EVD clamping during IHT in neurocritically ill cerebrovascular patients.
Methods
Study Center and Data Sources
A retrospective cohort study was conducted at Harborview Medical Center (HMC) using the Online Record of Clinical Activity (ORCA) and the electronic Anesthesia Information Management System (AIMS). Institutional review board approval was obtained from the University of Washington and the project was deemed appropriate as an institutional quality improvement project.
Harborview Medical Center is a 413-bed Level-I trauma center affiliated with the University of Washington and has a dedicated 30-bed neurocritical care unit (NICU) specialized in care for patients with stroke, traumatic brain and spinal cord injury, and neuraxial tumors. At HMC, all patients with indwelling EVD must be admitted to the NICU. Our protocol for transporting ICU patients mandates the presence of a registered ICU nurse (RN) to accompany patients at all times. If the patient is intubated, a respiratory therapist accompanies the RN. If the patient is transported for anesthesia care, an anesthesia provider will be present for the entire transport. We follow the American Association of Neuroscience Nurses practice guidelines for management of EVD and lumbar drains [19]. Thus, by default, all EVDs are expected to be clamped while in IHT. However, ICP monitoring is left to the discretion of the transport personnel. There is no protocol for a standardized testing tolerance to EVD clamping at HMC, and its use is left to the clinician’s discretion. EVD clamping in patients with SAH and ICH follows the conventional pattern of incremental increases in “setting of EVD.” As the setting of EVD changes throughout the hospital course, patient’s neurological condition, absolute and trend in ICP and serial evaluation of ventriculomegaly on non-contrast CT scan will be considered before decision for EVD removal or plans made for ventriculoperitoneal shunt.
Patient Population
Patients at least 18 years old who were diagnosed with nontraumatic intracranial hemorrhage and admitted to the NICU from September to December of 2015 were identified via daily chart screening at NICU admission. To be eligible, patients must have had an EVD placed within 24 h after admission and undergo at least one IHT from the NICU. We continued follow-up of all IHTs of enrolled patients until they were discharged from the NICU. Individual IHTs were excluded from analysis if the IHTs occurred without an EVD or if there were missing or unreliable ICP measures within 4 h prior to or after completion of IHT.
IHT Variables
IHT was classified for diagnostic or therapeutic procedures. IHTs for diagnostic procedures were defined as transports to CT, MRI, or angiography suite and included time spent during return of the patient to the NICU (round trips). IHTs for therapeutic procedures were defined as any transports from NICU to either the angiography suite or the operating room (one way trips) and did not include time spent during the procedure or return to the NICU since the EVD is normally reset and open to drain during the procedures.
The beginning of IHT was defined from the time the last NICU vital signs were recorded before departure for both diagnostic and therapeutic IHTs. For diagnostic procedures, the end of IHT was defined as the time the first vital signs were recorded following the patient’s return to the NICU. For therapeutic procedures, the end of IHT was defined as the first vital signs recorded at the final destination.
Outcomes
The main outcome of interest was incidence of intracranial hemodynamic complications associated with IHT, which were defined as an alteration in ICP and CPP after IHT. We categorized changes in ICP into three severity categories as follows: Category 1, ICP < 15 mmHg; Category 2, ICP 15–19 mmHg; and Category 3, ICP ≥ 20 mmHg. IHT associated with ICP change was defined as (1) post-IHT ICP ≥ 20 mmHg or (2) escalation into a higher ICP category. IHT associated with CPP complications was defined as post-IHT CPP < 50 mmHg.
We also examined factors associated with complications such as duration of IHT (10-min increments), number of days since admission when IHT took place, average hourly CSF output prior to IHT, EVD clamp status in NICU prior to IHT, unscheduled IHT, purpose of IHT, and intubated condition during IHT.
Data Collection
Clinical patient characteristics included age, gender, admission diagnosis, admission Glasgow Coma Scale (GCS) score, duration of EVD, presence of endotracheal intubation, hospital length of stay (LOS), ICU LOS, and discharge outcome. All IHTs from NICU in every patient were captured, and the following data were abstracted from the medical record and AIMS: purpose of IHT (diagnostic or therapeutic), destination (CT, MRI, Angiography, operating room), duration of IHT, vital signs, ICP and CPP both pre- and post-IHT, EVD clamp status, and CSF draining. EVD setting in NICU was classified as open or clamped. CSF drainage was measured in mL/h in the 24 h preceding the IHT.
Statistical Analysis
We tested the distributions of ICP, CPP, MAP and SBP, pre-IHT GCS score, days since NICU admission, duration of IHT, and pre-IHT ICP with the Kolmogorov–Smirnov test. Differences in median values and interquartile ranges (IQR) of those variables were then reported and evaluated using a Wilcoxon matched-pairs signed rank test for comparing pre- and post-IHT by patient and using the Wilcoxon rank sum test when comparing IHT characteristics by NICU EVD clamp status (open vs. clamped). Additional IHT characteristics (i.e., pre-IHT ICP ≥ 15 mmHg, IHT for diagnostic purposes, unscheduled IHT, intubation during IHT, and post-IHT ICP of ≥ 20 mmHg) were evaluated by comparing the characteristics of IHTs by EVD clamp status in the NICU and tested with a two-sample test for binomial proportions. Escalation of ICP category by EVD clamp status was tested with a Fisher exact test to compare categorical levels. Two-tailed statistical tests were considered significant at α < 0.05.
A multilevel mixed-effects Poisson regression was used to calculate the relative risk (RR) for transport characteristics associated with post-IHT ICP ≥ 20 mmHg. Ordinal logistic regression was used to calculate the odds ratios (OR) associated with increasing ICP category. Due to the small sample size, patient level characteristics (age, sex, diagnosis, and GCS score) were not included in the models, but both models were clustered by patient to account for individual level characteristics; individual IHTs were the unit of analysis. All RRs and ORs report the risk of those individual characteristics, adjusting for all other transport characteristics. As a secondary analysis, receiver operating characteristic (ROC) curves were constructed to test the ability of pre-IHT ICP and CSF drainage to predict both post-IHT ICP ≥ 20 mmHg and increasing ICP category. All analyses were performed using Stata version 13 [32].
Results
Characteristics of 19 Patients
During a 3-month period, 19 cerebrovascular patients with indwelling EVDs were admitted to the NICU. The most common admission diagnosis was SAH (79.0 %), the majority (ten patients) having high-grade SAH. Fourteen patients (73.7 %) were mechanically ventilated during at least one IHT. Seventy-one IHTs (39.9 %) occurred when the patients were intubated and on controlled mechanical ventilation. Patient details are presented in Table 1.
Characteristics of 178 IHTs
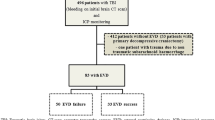
There were a total of 207 IHTs from NICU. We excluded 13 IHTs performed without indwelling EVD and 16 IHTs with missing pre- or post-IHT ICP. A total of 178 IHTs were included in the final analysis (Fig. 1).
EVD Characteristics
Of all 178 IHTs, 145 (81.5 %) had an open EVD clamp status in the NICU prior to IHT, and 33 (18.5 %) IHTs had clamped EVD status in 24 h before IHT. When actively drained, the mean hourly CSF output was 7.1 ± 3.8 mL/h.
Destination
Among the 50 unscheduled IHTs, 30 (60 %) were for an emergency CT scan and 10 (20 %) were for a therapeutic angiogram for treatment of cerebral vasospasm. While diagnostic IHTs were spread over the entire admission period while in the NICU, therapeutic IHTs occurred early in the NICU stay, in which 15 IHTs (41.7 %) occurred within the first three days. Median number of IHTs per patient was 8 (IQR 6–13). The most common IHT was to CT (median number of IHT per patient 5 [IQR 4–8].
Duration
Mean duration of diagnostic IHT was 50 ± 24 min, while that of therapeutic IHT was 26 ± 16 min. IHTs to MRI accounted for the longest IHT duration (66 ± 25 min) followed by diagnostic angiogram and CT (64 ± 32 and 46 ± 22 min, respectively). Mean duration of IHT for a therapeutic angiogram was 29 ± 17 min and to the operating room was 20 ± 12 min. Mean IHT duration was shorter in patients with lower pre-IHT GCS; for pre-IHT GCS score of 3–8, 9–13, and 14–15, mean IHT durations were 38 ± 19, 42 ± 22, and 57 ± 29 min, respectively.
IHT Complications
Median post-IHT ICP was higher compared to pre-IHT ICP (9 mmHg [5–14] vs. 7 mmHg [3–11], respectively, [p < 0.001]). Median post-IHT CPP was lower after therapeutic IHT (pre-IHT CPP of 74 (69–92) mmHg and post-IHT CPP 71 (50–82) mmHg, [p = <0.001]). However, there was no difference observed between pre- and post-CPP for diagnostic procedures. There was no difference between MAP and SBP before and after IHT for all IHTs and in the therapeutic subgroup (Table 2).
Pre- and post-IHT ICP remained below 15 mmHg in most IHTs (129, 72.5 %). Twenty-one IHTs (11.8 %) were, however, associated with a post-IHT ICP ≥ 20 mmHg. Among those, 14 IHTs (66.7 %) had newly developed intracranial hypertension associated with IHT. Six IHTs had post-IHT ICP elevated to more than 30 mmHg, five of which were observed in a single patient with high-grade SAH.
Thirty-three IHTs (18.5 %) were associated with escalation to a higher ICP category; 29 IHTs were from pre-ICP Category 1, and 4 IHTs were from pre-ICP Category 2. Cerebral hypoperfusion (CPP < 50 mmHg) occurred in 9 IHTs (5.1 %), with all occurring in patients without preexisting intracranial hypertension.
EVD Clamping Status Prior to IHT
Of 178 IHTs, 81.5 % IHTs (n = 145) were conducted while the EVDs were open and actively draining CSF in the NICU prior to IHT. Of these, forty IHTs (27.6 %) were associated with either a post-IHT ICP ≥ 20 mmHg or escalation of ICP category. None of the 33 IHTs (18.5 %) in patients whose EVD status was clamped in the NICU were associated with IHT-related ICP complications. The characteristics of IHT by EVD clamp status are presented in Table 3.
Factors Associated with IHT-Related ICP Complications
Factors associated with post-IHT ICP ≥ 20 mmHg were therapeutic IHT (adjusted relative risk [aRR] 5.82; 95 % CI, 1.76–19.19), pre-IHT ICP 15–19 mmHg (aRR 3.40; 95 % CI, 1.08–10.76), and post-IHT ICP > 20 mmHg (aRR 12.94; 95 % CI, 4.08–41.01). Therapeutic IHT was associated with escalation of ICP category (aOR 22.83; 95 % CI, 3.97–131.07).
Each 1 mL of average hourly CSF drained in the 24 h prior to IHT increased the risk of post-IHT ICP ≥ 20 mmHg by 11 % (aRR 1.11; 95 % CI, 1.01–1.23) and escalation of ICP category by almost 20 % (aOR 1.21; 95 % CI, 1.06–1.38). There was no association between ICP complications and the following IHT characteristics: number of days since ICU admission, unscheduled IHT, mechanical ventilation, and duration of IHT (Table 4).
Predictors of IHT Associated with Complications
Using generated ROC curves, we identified pre-IHT ICP ≥ 10 mmHg to be a good predictor for post-IHT ≥ 20 mmHg (area under the curve [AUC] = 0.84, SE = 0.05) with a sensitivity of 81.2 % and specificity of 25.2 %, and a fair predictor of escalation of ICP category (AUC = 0.72, SE = 0.05) with a sensitivity of 60.0 % and specificity of 22.1 %. Pre-IHT CSF drainage of ≥ 5 mL/h was found to be a poor predictor of post-IHT ICP ≥ 20 mmHg (AUC = 0.69, SE = 0.04) with a sensitivity of 95.2 % and specificity of 56.9 % and a poor predictor of ICP category escalation (AUC = 0.69, SE = 0.05) with a sensitivity of 82.6 % and specificity of 57.4 %.
Outcomes in 19 Patients
Fourteen patients (73.7 %) had at least one ICP complication. Patients with low-grade SAH (Hunt and Hess I–III) accounted for only four (9.3 %) of the total IHTs with post-IHT ICP ≥ 20 mmHg, and six IHTs (13.9 %) had escalation of ICP category; while the high-grade group (Hunt and Hess IV–V) accounted for 15 IHTs (14.9 %) with post-IHT ≥ 20 mmHg, and 21 IHTs (20.8 %) had escalation of ICP category. Five patients in our study died during hospitalization. These five patients accounted for 47 IHTs; 10 IHTs (21.3 %) had post-IHT ≥ 20 mmHg and 10 IHTs (21.3 %) had escalation of ICP category. Among the 14 patients who were alive at discharge, 11 IHTs (8.4 %) had post-IHT ≥ 20 mmHg and 23 IHTs (17.6 %) had escalation of ICP category.
Discussion
The main findings of this study are that (1) neurocritically ill cerebrovascular patients experienced significantly large number of IHTs, especially for head CT scans; (2) IHTs occurred in the more critically ill patients and early, during the first few days after NICU admission; (3) the first testing for tolerance to EVD clamping often occurred during IHT and predisposed patients to ICP complications; and (4) factors associated with IHT-related complications were pre-IHT EVD clamp status, pre-IHT ICP, hourly EVD CSF output, and IHT purpose. These findings suggest that the practice of routine EVD clamping for IHT in neurocritically ill patients should be reconsidered.
When assessing patients whose EVDs are actively drained in the NICU, our study shows that clinicians need to consider the purpose of IHT (diagnostic vs. therapeutic), baseline pre-IHT ICP, and the amount of CSF output prior to IHT. In our study, those patients transported for therapeutic procedures were more critically ill at baseline with higher baseline pre-IHT ICP and lower pre-IHT CPP, thus predisposing them to either post-IHT ICP ≥ 20 mmHg or escalation of ICP category. The findings of elevated baseline ICP ≥ 15 mmHg are consistent with that of Picetti et al. [9], who prospectively observed neurocritically ill patients being transported to the CT scanner and found that ICP remained high during IHTs of all patients with pre-IHT ICP > 20 mmHg. In these patients with elevated baseline ICP, further increase in ICP could be prevented by treatment of intracranial hypertension within 2 h prior to IHT [6]. One recent study demonstrated that a mean 24-h CSF output of >130 mL, at an EVD setting of +15 mmHg, was associated with EVD clamp trial failure in SAH patients during the EVD weaning period [33]. While this study was not conducted within the context of IHT, the findings suggest some patients with high hourly CSF output rely on external CSF diversion. Given these three risk factors, patients at high risk of ICP complications may benefit from continuing CSF diversion during IHT in addition to aggressive intracranial hypertension treatment prior to IHT. These three risk factors may also help prioritize ICP monitoring in resource-limited environments.
In this study, a large number of IHTs of neurocritically ill patients with indwelling EVDs were associated with ICP complications. Loss of cerebral autoregulation, changes in patient’s position, and altered ventilation make these patients more susceptible to CPP effects including cerebral ischemia during IHT. Since the reduction in CPP in our project was primarily related to elevation in ICP, it might stand to reason that ICP should be routinely monitored and recorded during IHT. However, while prospective studies include meticulous ICP and vital sign monitoring and recording, documentation of ICP or, for that matter, any of the vital signs during IHT on paper or in the electronic medical record is not standard of care in real-world practice. In a prior study assessing an implementation of a standardized evaluation plan for IHTs with a specialized team for transporting critically ill patients, documentation of transport data did not occur 15.5 % of the time [34]. This illustrates the challenges of consistent data collection. These missing data during a vulnerable period are not only problematic for patient care but also underestimate the actual incidence of IHT-related complications, resulting in delayed recognition and delayed treatment of intracranial hypertension. Other than administration of hyperosmolar agents and/or transient hyperventilation, unclamping of the EVD to allow CSF drainage may also be a therapeutic option. Therefore, monitoring and documentation of ICP and other vital signs during IHT in neurocritically ill patients with indwelling EVD is warranted.
In this cohort, ICP complications during IHT occurred only in those patients whose EVD was being actively drained in the NICU prior to IHT and then clamped for and during IHT. This finding is important because many patients in their early NICU stay are dependent on CSF diversion to maintain normal ICP, especially early in their hospital course when many IHTs occur. Routine EVD clamping for IHT in these patients results in a testing for tolerance to EVD clamping, which according to this study, they fail during IHT. Present data suggest that tolerance to EVD clamping should be tested in the NICU prior to deciding on whether or not to clamp EVDs for IHT.
Current national guidelines on EVD management during IHT do not address testing for tolerance to EVD clamping and are driven largely by concerns of CSF overdrainage. It is also unknown to what extent the tests are routinely performed in NICUs across the USA, and it is unclear as to how test for tolerance to EVD clamping is defined. Bérubé et al. [35] defined ICP monitoring and presence of EVD as risk factors for transport-related complications and suggested evaluating the patients in dorsal decubitus position 10 min before leaving the unit. While a significant proportion of IHTs are unscheduled and cannot be avoided, it is still prudent to understand risks involved with these IHTs. We think that testing for tolerance to EVD clamping should probably replicate IHT conditions and destination characteristics. Potential elements to be considered include addressing head-of-bed positioning, simulated time of IHT, vertical positioning of EVD chambers, ICP targets and CSF volume for opening and clamping EVD during IHT, and monitoring of ICP. In a prospective observational study, 26 % of the IHTs of patients with ICP monitoring required additional ICP therapy during IHT [22]. According to the data, patients at high risk may require the transport personnel to carry medications such as sedation or hyperosmolar agents to treat high ICP that potentially occurs during IHT. Furthermore, EVD management must occur in the context of physiology that affects ICP, including targets for PaCO2, and blood pressure. Since longer head-of-bed to zero-positioning periods such as occur with MRI and diagnostic angiograms may not be tolerated in patients with poor intracranial compliance despite open EVD and active CSF drainage, the risk benefit ratio of the IHT should be considered and reassessed. Our study suggests that patients whose EVD is clamped in the NICU without ICP complications prior to IHT may be able to tolerate IHT without additional risk. Regardless of tolerance to EVD clamping in the NICU, however, ICP monitoring during IHT and at the final destinations should follow similar monitoring and documentation standards as they would in the NICU.
One of the strengths of our study is the fact that 178 IHTs were studied, thus making it one of the largest IHT studies on neurocritically ill patients. This gave us the opportunity to cluster the results at a patient level to identify risk factors for post-IHT ICP complications. Some limitations of this study are its retrospective single-center design and small patient sample size, which may limit study generalizability. We were unable to collect real-time IHT data on hemodynamic and ICP monitoring, actual EVD management during IHT (clamped or open), actual duration of EVD clamping, and precise IHT duration. Since changes in ICP can be multifactorial (head-of-bed positioning, pain, agitation, hypoxia, hypercarbia, and hypocarbia), contributions of such factors during IHT in addition to effect of clamping of EVD on ICP could not be determined. Moreover, we were unable to demonstrate duration of ICP elevation or its effect on cerebral O2 delivery since none of our patients received PbtO2 monitoring. We were not able to include some patient factors such as age, sex, diagnosis, admission GCS, and survival outcomes into a multivariate analysis model for risk factors associated with complications due to small sample size, although IHTs were clustered by patient to control for these individual characteristics. In addition, the majority of the patients had a SAH, which may limit generalization of our findings to other neurocritically ill patients.
In summary, results from this small study provide new and clinically relevant information, suggesting that routine EVD clamping for IHT in neurocritically ill cerebrovascular patients is associated with post-IHT ICP ≥ 20 mmHg and escalation in ICP category. Findings also suggest that a formal approach to evaluating the potential for ICP complications during IHT such as by testing tolerance to EVD clamping and simulating IHT conditions is warranted. Finally, all neurocritically ill cerebrovascular patients with indwelling EVD should receive ICP monitoring during IHT.
References
Gillman L, Leslie G, Williams T, Fawcett K, Bell R, McGibbon V. Adverse events experienced while transferring the critically ill patient from the emergency department to the intensive care unit. Emerg Med J. 2006;23:858–61.
Lahner D, Nikolic A, Marhofer P, et al. Incidence of complications in intrahospital transport of critically ill patients—experience in an Austrian university hospital. Wien Klin Wochenschr. 2007;119:412–6.
Zuchelo LT, Chiavone PA. Intrahospital transport of patients on invasive ventilation: cardiorespiratory repercussions and adverse events. J Bras Pneumol. 2009;35:367–74.
Parmentier-Decrucq E, Poissy J, Favory R, et al. Adverse events during intrahospital transport of critically ill patients: incidence and risk factors. Ann Intensive Care. 2013;3:10.
Jia L, Wang H, Gao Y, Liu H, Yu K. High incidence of adverse events during intra-hospital transport of critically ill patients and new related risk factors: a prospective, multicenter study in China. Crit Care. 2016;20:12.
Andrews PJ, Piper IR, Dearden NM, Miller JD. Secondary insults during intrahospital transport of head-injured patients. Lancet. 1990;335:327–30.
Kalisch BJ, Kalisch PA, Burns SM, Kocan MJ, Prendergast V. Intrahospital transport of neuro ICU patients. J Neurosci Nurs. 1995;27:69–77.
Papson JP, Russell KL, Taylor DM. Unexpected events during the intrahospital transport of critically ill patients. Acad Emerg Med. 2007;14:574–7.
Picetti E, Antonini MV, Lucchetti MC, et al. Intra-hospital transport of brain-injured patients: a prospective, observational study. Neurocrit Care. 2013;18:298–304.
Rosenbaum BP, Vadera S, Kelly ML, Kshettry VR, Weil RJ. Ventriculostomy: frequency, length of stay and in-hospital mortality in the United States of America, 1988–2010. J Clin Neurosci. 2014;21:623–32.
Woodward S, Addison C, Shah S, Brennan F, MacLeod A, Clements M. Benchmarking best practice for external ventricular drainage. Br J Nurs. 2002;11:47–53.
Slazinski T, Anderson TA, Cattel E, et al. Care of the patient undergoing intracranial pressure monitoring/external ventricular drainage or lumbar drainage. Glenview, IL: American Association of Neuroscience Nurses; 2011. p. 1–38.
Tu H. Intrafacility transportation of patients with acute brain injury. J Neurosci Nurs. 2014;46:E12–6.
Hasan D, Lindsay KW, Vermeulen M. Treatment of acute hydrocephalus after subarachnoid hemorrhage with serial lumbar puncture. Stroke. 1991;22:190–4.
Kasuya H, Shimizu T, Kagawa M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage: a retrospective analysis of 108 patients. Neurosurgery. 1991;28:56–9.
Hellingman CA, van den Bergh WM, Beijer IS, et al. Risk of rebleeding after treatment of acute hydrocephalus in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:96–9.
Cohen-Gadol AA. Remote contralateral intraparenchymal hemorrhage after overdrainage of a chronic subdural hematoma. Int J Surg Case Rep. 2013;4:834–6.
Niimura M, Takai K, Taniguchi M. Postoperative epidural haematomas associated with hydrocephalus caused by intraoperative overdrainage of cerebrospinal fluid: two case reports with a literature review of 19 cases. BMJ Case Rep. 2015. doi:10.1136/bcr-2014-206654.
Salunke P, Savardekar A, Chhabra R, Mathuriya SN. Subdural collections complicating third ventriculostomy: over-drainage or failure of ventriculostomy? Neurol India. 2012;60:254–5.
Wang QP, Zhou ZM, You C. Paradoxical herniation caused by cerebrospinal fluid drainage after decompressive craniectomy. Neurol India. 2014;62:79–80.
Doring BL, Kerr ME, Lovasik DA, Thayer T. Factors that contribute to complications during intrahospital transport of the critically ill. J Neurosci Nurs. 1999;31:80–6.
Kleffmann J, Pahl R, Deinsberger W, Ferbert A, Roth C. Intracranial pressure changes during intrahospital transports of neurocritically ill patients. Neurocrit Care. 2016. doi:10.1007/s12028-016-0274-6.
Warren J, Fromm RE Jr, Orr RA, Rotello LC, Horst HM, American College of Critical Care Medicine. Guidelines for the inter- and intrahospital transport of critically ill patients. Crit Care Med. 2004;32:256–62.
Ferdinande P. Recommendations for intra-hospital transport of the severely head injured patient. Working Group on Neurosurgical Intensive Care of the European Society of Intensive Care Medicine. Intensive Care Med. 1999;25:1441–3.
Recommendations for the Safe Transfer of Patients with Brain Injury. London: The Association of Anaesthetists of Great Britain and Ireland. 2006. https://www.aagbi.org/sites/default/files/braininjury.pdf. Accessed 22 Oct 2015.
Jarden RJ, Quirke S. Improving safety and documentation in intrahospital transport: development of an intrahospital transport tool for critically ill patients. Intensive Crit Care Nurs. 2010;26:101–7.
Fanara B, Manzon C, Barbot O, Desmettre T, Capellier G. Recommendations for the intra-hospital transport of critically ill patients. Crit Care. 2010;14:R87.
Guidelines for transport of critically ill patients. Melbourne: Australasian college for emergency medicine, Australian and New Zealand college of anaesthetists and college of intensive care medicine of Australia and New Zealand. 2015. https://www.acem.org.au/getattachment/a7701840-29f2-4096-872a-b213bec75d9d/P03-Guidelines-for-Transport-of-Critically-Ill-Pat.aspx. Accessed 22 Oct 2015.
Quenot JP, Milesi C, Cravoisy A, et al. Intrahospital transport of critically ill patients (excluding newborns) recommendations of the Societe de Reanimation de Langue Francaise (SRLF), the Societe Francaise d’Anesthesie et de Reanimation (SFAR), and the Societe Francaise de Medecine d’Urgence (SFMU). Ann Intensive Care. 2012;2:1.
Whiteley S, Macartney I, Mark J, Barratt H, Binks R. Guidelines for the transport of the critically ill adult (3rd edition). The Intensive Care Society. 2011. http://www.ics.ac.uk/EasysiteWeb/getresource.axd?AssetID=482. Accessed 22 Oct 2015.
Fried HI, Nathan BR, Rowe AS, et al. The insertion and management of external ventricular drains: an evidence-based consensus statement: a statement for healthcare professionals from the neurocritical care society. Neurocrit Care. 2016;24:61–81.
StataCorp. Stata statistical software release 13.1. College Station: StataCorp LP; 2013.
Zolal A, Juratli T, Dengl M, Ficici KH, Schackert G, Sobottka SB. Daily drained CSF volume is a predictor for shunt dependence—A retrospective study. Clin Neurol Neurosurg. 2015;138:147–50.
Jones HM, Zychowicz ME, Champagne M, Thornlow DK. Intrahospital transport of the critically ill adult—a standardized Evaluation Plan. Dimens Crit Care Nurs. 2016;35:133–46.
Bérubé M, Bernard F, Marion H, Parent J, Thibault M, Williamson DR, Albert M. Impact of a preventive programme on the occurrence of incidents during the transport of critically ill patients. Intensive Crit Care Nurs. 2013;29:9–19.
Acknowledgments
This study was conducted at Harborview Medical Center, University of Washington, Seattle, WA.
Financial Support
No institutional or departmental funds were used for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have disclosed no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Chaikittisilpa, N., Lele, A.V., Lyons, V.H. et al. Risks of Routinely Clamping External Ventricular Drains for Intrahospital Transport in Neurocritically Ill Cerebrovascular Patients. Neurocrit Care 26, 196–204 (2017). https://doi.org/10.1007/s12028-016-0308-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0308-0