Abstract
Background
MicroRNAs (miRNAs) are small, non-coding RNAs that are involved in carcinogenesis through posttranscriptional gene regulatory activity. The current study aimed to evaluate serum miR-21 expression levels as potential biomarkers for diagnosis and prognosis of colorectal cancer (CRC) patients.
Methods
Quantitative real-time RT-PCR was applied to determine the relative expression level of miR-21 in serum. At the same time, the sensitivity and specificity of this marker were evaluated by receiver operating characteristic (ROC) curve analysis.
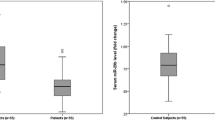
Results
miR-21 expression levels of serum were 3.4 and 1.25 in patient and control, respectively (p < 0.05). The sensitivity and specificity of miR-21 were found to be 95.8% and 91.7%, respectively. The high expression level of serum miR-21 were associated with higher local recurrence, TNM staging, PT staging, venous invasion, liver metastasis, and recurrence (p < 0.05).
Conclusion
The results of this study indicated that miR-21 expression levels in serum can be considered as a novel non-invasive biomarker for early detection and prognosis of CRC patients.




Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(74–108):3.
El-Bolkainy TN, ASakr M, Nouh AA, El-Din NH. A comparative study of rectal and colonic carcinoma: demographic, pathologic and TNM staging analysis journal of the Egyptian. Natl Cancer Inst. 2006;18:258–63.
Mastalier B, Botezatu C. Colentina surgical clinic experience in treatment of rectal cancer. Romanian statistical review - supliment trimestrul II. 2012:126–34.
Minna J, Schiller JH (2008): Harrison’s principles of internal medicine. New York: McGraw-Hill, 285(16): 12011–12027.
Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, et al., editors. SEER Cancer Statistics Review, 1975–2011. Bethesda: National Cancer Institute; 2014. p. 2014.
Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009. Am J Gastroenterol. 2009;104:739–50.
Qaseem A, Denberg TD, Hopkins RH Jr, Humphrey LL, Levine J, Sweet DE, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–86.
Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US multi-society task force on colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95.
Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J M. 1993;328:1365–71.
Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706.
Fakih MG, Padmanabhan A. CEA monitoring in colorectal cancer.What you should know. Oncology (Williston Park). 2006;20:579–87.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66.
Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69.
Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer. 2007;43:1529–44.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8.
Chevillet JR, Lee I, Briggs HA, He Y, Wang K. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules. 2014;19:6080–105.
Kanaan Z, Rai SN, Eichenberger MR, Roberts H, Keskey B, Pan J, et al. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012;256:544–51.
Mishra PJ. Non-coding RNAs as clinical biomarkers for cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2014;14:917–9.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8.
Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PLoS One. 2011;6:e20769.
Liu GH, Zhou ZG, Chen R, Wang MJ, Zhou B, Li Y, et al. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumor Biol. 2013;34(4):2175–81.
Ma Y, Zhang P, Yang J, Liu Z, Yang Z, Qin H. Candidate microRNA biomarkers in human colorectal cancer: systematic review profiling studies and experimental validation. Int J Cancer. 2012;130(9):2077–87.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17.
Bresalier RS, Kopetz S, Brenner DE. Blood-based tests for colorectal cancer screening: do they threaten the survival of the FIT test? Dig Dis Sci. 2015;60(3):664–71.
Toiyama Y, Takahashi M, Hur K, Nagasaka T, Tanaka K, Inoue Y, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013;105(12):849–59.
Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013;329(2):125–36.
Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–26.
Bastaminejad S, Taherikalani M, Ghanbari R, Akbari A, Shabab N, Saidijam M. Investigation of microRNA-21 expression levels in serum and stool as a potential non-invasive biomarker for diagnosis of colorectal cancer. Iran Biomed J. 2016;21:106–13.
Nedaeinia R, Sharifi M, Avan A, Kazemi M, Rafiee L, Ghayour-Mobarhan M, et al. Locked nucleic acid anti-miR-21 inhibits cell growth and invasive behaviors of a colorectal adenocarcinoma cell line: LNA-anti-miR as a novel approach. Cancer Gene Ther. 2016;23:246–53.
Almeida AL, Bernardes MV, Feitosa MR, Peria FM, Tirapelli DP, Rocha JJ, et al. Serological under expression of microRNA-21, microRNA-34a and microRNA-126 in colorectal cancer. Acta Cir Bras. 2016;31:13–8.
Montagnana M, Benati M, Danese E, Minicozzi AM, Paviati E, Gusella M, et al. (2016): plasma expression levels of circulating miR-21 are not useful for diagnosing and monitoring colorectal cancer. Clin Lab. 2016;62:967–70.
Menendez P, Padilla D, Villarejo P, Palomino T, Nieto P, Menendez JM, et al. Prognostic implications of serum microRNA-21 in colorectal cancer. J Surg Oncol. 2013;108:369–73.
Xi Y, Shalgi R, Fodstad O, et al. Differentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancer. Clin Cancer Res. 2006;12:2014–24.
Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, et al. Altered expression of miR-21, miR-31, miR-143m and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402.
Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez MLA, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One. 2011;6:e16979.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghareib, A.F., Mohamed, R.H., Abd el-Fatah, A.R. et al. Assessment of Serum MicroRNA-21 Gene Expression for Diagnosis and Prognosis of Colorectal Cancer. J Gastrointest Canc 51, 818–823 (2020). https://doi.org/10.1007/s12029-019-00306-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-019-00306-w