Abstract
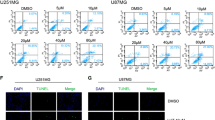
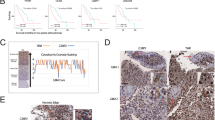
High-grade gliomas are refractory to the current mode of treatment primarily due to their inherent resistance to cell death. Tamoxifen has been reported to inhibit growth and induce cell death of glioma cells in vitro, in an estrogen-receptor-independent manner. Delineating the molecular mechanism underlying tamoxifen-induced cell death of human glioma cells would help in identifying pathways/genes that could be targeted to induce tumor-cell-specific cell death. In the present study, tamoxifen was found to bring about autophagic cell death of human glioma cells that was accompanied by oxidative stress induction, JNK activation, downregulation of anti-autophagic BCL2 family members, viz. BCL2 and BCL-XL, and increased expression of the pro-autophagic members BCL-Xs and BAK. Oxidative stress induction appears to be primarily responsible for the tamoxifen-induced cell death since the cell death, JNK activation, and the alterations in the expression levels of BCL2 family members were abrogated on pretreatment with antioxidant vitamin E. MiR-21, an oncogenic miRNA, is known to be highly upregulated in malignant glioma. Inhibition of miR-21 activity was found to enhance tamoxifen-induced cell death of U87 MG malignant glioma cells. Tamoxifen treatment coupled with miR-21 inhibition could therefore be an effective strategy for the treatment of malignant gliomas.





Similar content being viewed by others
References
Adams JM, Cory S (2007) Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol 19:488–496
Adamson C, Kanu OO, Mehta AI et al (2009) Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 18:1061–1083
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2008) Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15:171–182
Cloughesy TF, Woods RP, Black KL, Couldwell WT, Law RE, Hinton DR (1997) Prolonged treatment with biologic agents for malignant glioma: a case study with high dose tamoxifen. J Neurooncol 35:39–45
Couldwell W, Hinton D, He S et al (1994) Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS Lett 345:43–46
Di Cristofori A, Carrabba G, Lanfranchi G, Menghetti C, Rampini P, Caroli M (2013) Continuous tamoxifen and dose-dense temozolomide in recurrent glioblastoma. Anticancer Res 33:3383–3389
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Gabriely G, Wurdinger T, Kesari S et al (2008) MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol 28:5369–5380
Gwak HS, Kim TH, Jo GH et al (2012) Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One 7, e47449
Hardwick JM, Soane L (2013) Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol 5
Hong L, Han Y, Zhang Y et al (2013) MicroRNA-21: a therapeutic target for reversing drug resistance in cancer. Expert Opin Ther Targets 17:1073–1080
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18:571–580
Klionsky DJ, Abdalla FC, Abeliovich H et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Kohli L, Kaza N, Coric T et al (2013) 4-Hydroxytamoxifen induces autophagic death through K-Ras degradation. Cancer Res 73:4395–4405
Krichevsky AM, Gabriely G (2009) miR-21: a small multi-faceted RNA. J Cell Mol Med 13:39–53
Mandlekar S, Yu R, Tan T-H, Kong A-NT (2000) Activation of caspase-3 and c-Jun NH2-terminal kinase-1 signaling pathways in tamoxifen-induced apoptosis of human breast cancer cells. Cancer Res 60:5995–6000
Moodbidri MS, Shirsat NV (2005) Activated JNK brings about accelerated apoptosis of Bcl-2-overexpressing C6 glioma cells on treatment with tamoxifen. J Neurochem 92:1–9
Osborne CK (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339:1609–1618
Papagiannakopoulos T, Shapiro A, Kosik KS (2008) MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res 68:8164–8172
Pollack IF, Randall MS, Kristofik MP, Kelly RH, Selker RG, Vertosick FT Jr (1990) Effect of tamoxifen on DNA synthesis and proliferation of human malignant glioma lines in vitro. Cancer Res 50:7134–7138
Robins HI, Won M, Seiferheld WF et al (2006) Phase 2 trial of radiation plus high-dose tamoxifen for glioblastoma multiforme: RTOG protocol BR-0021. Neuro Oncol 8:47–52
Seca H, Lima RT, Lopes-Rodrigues V, Guimaraes JE, Almeida GM, Vasconcelos MH (2013) Targeting miR-21 induces autophagy and chemosensitivity of leukemia cells. Curr Drug Targets 14:1135–1143
Tang P, Roldan G, Brasher PM et al (2006) A phase II study of carboplatin and chronic high-dose tamoxifen in patients with recurrent malignant glioma. J Neurooncol 78:311–316
The Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Acknowledgments
This work was supported by a grant from the Indian Council of Medical Research (N.V.S.), a fellowship from the Council of Scientific and Industrial Research (M.H.), and a fellowship from the University Grants Commission (S.U), India.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mugdha Harmalkar and Shailendra Upraity contributed equally to this work.
Rights and permissions
About this article
Cite this article
Harmalkar, M., Upraity, S., Kazi, S. et al. Tamoxifen-Induced Cell Death of Malignant Glioma Cells Is Brought About by Oxidative-Stress-Mediated Alterations in the Expression of BCL2 Family Members and Is Enhanced on miR-21 Inhibition. J Mol Neurosci 57, 197–202 (2015). https://doi.org/10.1007/s12031-015-0602-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0602-x