Abstract
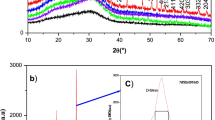
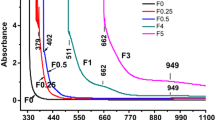
The relationship among the composition, structure and selected properties for five series of silver phosphate glasses containing 0, 5, 10, 15 and 20 wt% Fe2O3 has been investigated. The synthesized glasses have been characterized using different experimental techniques. X-ray diffraction studies revealed that the glasses are amorphous in nature. IR spectral studies have shown the presence of characteristic P–O–P linkages of linear phosphate chains, presence of O–P–O units in the phosphate tetrahedral and the formation of P–O–Fe bonds in the doped glass. It is also confirmed that due to doping of Fe2O3, loosening of glassy structure occurred and the glass became more disordered. Differential scanning calorimetric (DSC) studies revealed that glass transition temperature increased with Fe2O3 concentration. Scanning electron microscopic studies have shown that Fe2O3 doping modifies the microstructures of the glass and at lower concentration of dopant, a nanostructure is obtained. Electrical conductivity measurements from 303 to 373 K in a frequency range from 100 Hz to 5 MHz have indicated that all glasses are ionic conductors with Ag+ ions as the charge carrier. Fe2O3 doping in silver phosphate glass increased the electrical conductivities. Results have shown that dielectric constants increased with the increase of temperature at all the frequencies; a.c. and d.c. conductivities have been separated and a Cole–Cole plot is also drawn. Dielectric losses in all the glasses decreased with frequency at a particular temperature. It is found that Ag2O–P2O5 glass doped with 5 wt% Fe2O3 gives high OCV value and the doped glass can be used as an electrolyte for solid-state batteries.













Similar content being viewed by others
References
Tiwari B, Dixit A, Kothiyal G P, Pandey M and Deb S K 2007 BARC Newslett. 285 167
Brow R K 2000 J. Non-Cryst. Solids 263–264 1
Garbarczyk J E, Pietrzak T K, Wasiucionek M, Kaleta A, Dorau A and Nowinski J L 2015 Solid State Ion. 272 53
Baia L, Muresan D, Baia M, Popp J and Simon S 2007 Vibr. Spectrosc. 43 313
Kabi S and Ghosh A 2014 Solid State Ion. 262 778
Rao G V and Shashikala H D 2014 J. Non-Cryst. Solids 402 204
Choudhary B P and Singh N B 2014 Emerg. Mater. Res. 3 70
Singh N B and Chaudhary B P 2015 J. Therm. Anal. Calorim. 120 1217
Garbarczyk J E, Wasiucionek M, Jozwiak P, Nowinski J L and Julien C M 2009 Solid State Ion. 180 531
Das S S, Baranwal B P, Gupta C P and Singh P 2005 Indian J. Eng. Mater. Sci. 12 58
Das S S, Baranwal B P, Gupta C P and Singh P 2003 J. Power Sources 114 346
Das S S, Srivastava P K, Singh N P and Srivastava V 2010 Indian J. Eng. Mater. Sci. 17 123
Thangadurai V, Huggins R A and Weppner W 2002 J. Power Sources 108 64
Santic A, Kim C W, Day D E and Mogus-Milankovic A 2010 J. Non-Cryst. Solids 356 2699
Mugoni C, Montorsi M, Siligardi C and Jain H 2014 J. Non-Cryst. Solids 383 137
Almond D P, Hunter C C and West A R 1984 J. Mater. Sci. 19 3236
Jonscher A K 1977 Nature 267 673
Funke K 1993 Prog. Solid State Chem. 22 111
Acknowledgements
We are grateful to Prof. B Middendorf, University Kassel, Germany, for recording X-ray diffraction and SEM pictures. We acknowledge the financial support in the form of a research project from DRDO, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
CHOUDHARY, B.P., SINGH, N.B. Characterization of Fe3+-doped silver phosphate glasses. Bull Mater Sci 39, 1651–1663 (2016). https://doi.org/10.1007/s12034-016-1329-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1329-1