Abstract
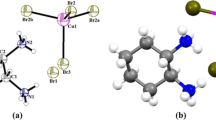
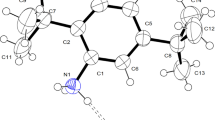
The new organic–inorganic hybrid compound [C6H6NO2]2CuCl4⋅H2O has been grown from an aqueous mixture by the solvent evaporation method. X-ray diffraction, Hirshfeld surface analysis, FT-IR and FT-Raman spectroscopy were applied to characterize the composition and crystal structure of the complex. It is crystallized in a triclinic system (\( {P}\bar {{1}}\) space group). The structure of this compound might be described as layered with two parallel anionic and cationic layers. The first layer is composed of isolated square planar [CuCl4]2− and the second layer of [C6H6NO2]2+. The water molecule is placed between the layers formed by organic cations along the b axis. Network hydrogen-bonding and π–π interactions lead to the formation of a three-dimensional architecture. Hirshfeld surface analysis for visually analysing intermolecular interactions in crystal structures employing molecular surface contours and 2D fingerprint plots has been used to scrutinize molecular shapes. The vibration properties of this structure were studied by IR spectroscopy and Raman scattering. Vibration spectra were also calculated theoretically by means of Gaussian 03 package of programs within the density functional theory (DFT) framework using the B3LYP/LanL2DZ level of theory. To sum up, good consistency is found between the calculated results on one hand and the IR and Raman spectra and experimental structure on the other. This study confirms the presence of the organic cations [C6H6NO2]2+, the inorganic anions [CuCl4]2− and H2O.









Similar content being viewed by others
References
Hajlaoui S, Chaabane I, Oueslati A, Guidara K and Bulou A 2014 Spectrochim. Acta A 117 225
Chaabane I, Hlel F and Guidara K 2008 J. Alloys Compd. 461 495
Ben Bechir M, Karoui K, Tabellout M, Guidara K and Ben Rhaiem A 2014 J. Alloys Compd. 588 551
Karâa N, Hamdi B, Salah A and Zouari R 2012 J. Mol. Struct. 1013 168
Willett R D and Twamley B 2001 Acta Crystallogr. 57 573
Kurawa M A, Adams C J and Orpen A G 2008 Acta Crystallogr., E 64 924
Swamy G Y S K, Ravikumar K and Ramakrishna K V S 2013 Polyhedron 49 145
Bhattacharya R, Ray M S, Dey R, Righi L, Bocelli G and Ghosh A 2002 Polyhedron 21 2561
Farrugia L J 1999 J. Appl. Crystallogr. 32 837
Sheldrick G M 1986 SHELXS-86 program for crystal structure solution (Germany: University of Gottingen)
Sheldrick G M 1997 SHELXL-97 program for crystal structure refinement (Germany: University of Gottingen)
Farrugia L J 1997 J. Appl. Crystallogr. 30 565
Brandenburg K 1998 Diamond Version 2.0 (Bonn, Germany: Impact GbR)
Wolff S K, Grimwood D J, McKinnon J J, Turner M J, Jayatilaka D and Spackman M A 2013 Crystal Explorer Version 3.1 (Perth: University of Western Australia)
Seth S K, Saha N C, Ghosh S and Kar T 2011 Chem. Phys. Lett. 506 309
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R et al 2004 Gaussian 03, Revision C 02 (Wallingford: Gaussian Inc)
Zhu R Q 2011 Acta Crystallogr. E 67 112
Elangovan A, Thamaraichelvan A, Ramu A, Athimoolamc S and Natarajan S, Acta Crystallogr. E 63 224
Karâa N, Hamdi B, Oueslati A, Salah A and Zouari R 2010 J. Inorg. Organomet. Polym. 20 746
Hajlaoui S, Chaabane I, Oueslati A, Guidara K and Bulou A. 2014, Spectrochim. Acta A 117 225
Chen R H, Chen Y C, Shern C S and Fukami T 2009 J. Solid State Ion. 180 356
Acknowledgements
We would like to thank the members of units of common services, in particular Mr Tarek Gargouri, at the University of Sfax for their assistance in the measurements of X-ray diffraction. We are also thankful to Prof Hamadi Khemakhem for co-operating in the Raman spectroscopy measurement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic Supplementary Material
Crystallographic data for the title compound, have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication CCDC 1046854. Copies of these data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: + 44(0)-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
Rights and permissions
About this article
Cite this article
AZOUZI, K., HAMDI, B., ZOUARI, R. et al. Synthesis, structure and Hirshfeld surface analysis, vibrational and DFT investigation of (4-pyridine carboxylic acid) tetrachlorocuprate (II) monohydrate. Bull Mater Sci 40, 289–299 (2017). https://doi.org/10.1007/s12034-017-1375-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-017-1375-3