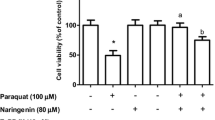
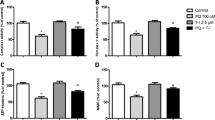
Abstract
Carnosic acid (CA) is a phenolic diterpene obtained from Rosmarinus officinalis L. and has demonstrated cytoprotective properties in several experimental models. CA exerts antioxidant effects by upregulating the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which controls the expression of antioxidant and phase II detoxification enzymes. Heme oxygenase-1 (HO-1) expression is modulated by Nrf2 and has been demonstrated as part of the mechanism underlying the CA-induced cytoprotection. Nonetheless, it remains to be studied whether and how HO-1 would mediate CA-elicited anti-inflammatory effects. Therefore, we have investigated here whether and how CA would prevent paraquat (PQ)-induced inflammation-related alterations in human neuroblastoma SH-SY5Y cells. SH-SY5Y cells were pretreated for 12 h with CA at 1 μM before exposure to PQ for further 24 h. CA suppressed the PQ-induced alterations on the levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and cyclooxygenase-2 (COX-2) through a mechanism involving the activation of the Nrf2/HO-1 axis. Furthermore, we observed a crosstalk between the Nrf2/HO-1 signaling pathway and the activation of the nuclear factor-κB (NF-κB) transcription factor, since administration of ZnPP IX (specific inhibitor of HO-1) or Nrf2 knockdown using small interfering RNA (siRNA) abolished the anti-inflammatory effects induced by CA. Moreover, administration of SN50 (specific inhibitor of NF-κB) inhibited the PQ-induced inflammation-related effects in SH-SY5Y cells. Therefore, CA exerted anti-inflammatory effects in SH-SY5Y cells through an Nrf2/HO-1 axis-dependent manner associated with downregulation of NF-κB.







Similar content being viewed by others
References
de Oliveira MR (2016) The dietary components carnosic acid and carnosol as neuroprotective agents: a mechanistic view. Mol Neurobiol 53:6155–6168. doi:10.1007/s12035-015-9519-1
Bahri S, Jameleddine S, Shlyonsky V (2016) Relevance of carnosic acid to the treatment of several health disorders: molecular targets and mechanisms. Biomed Pharmacother 84:569–582. doi:10.1016/j.biopha.2016.09.067
Maione F, Cantone V, Pace S, Chini MG, Bisio A, Romussi G, Pieretti S, Werz O et al (2016) Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br J Pharmacol. doi:10.1111/bph.13545
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39:199–218. doi:10.1016/j.tibs.2014.02.002
Esteras N, Dinkova-Kostova AT, Abramov AY (2016) Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biol Chem 397:383–400. doi:10.1515/hsz-2015-0295
Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X (2016) Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. doi:10.1007/s12035-016-0111-0
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. doi:10.1016/j.bbagen.2012.09.008
Nakagami Y (2016) Nrf2 is an attractive therapeutic target for retinal diseases. Oxidative Med Cell Longev 2016:7469326. doi:10.1155/2016/7469326
Brown KK, Hampton MB (2011) Biological targets of isothiocyanates. Biochim Biophys Acta 1810:888–894. doi:10.1016/j.bbagen.2011.06.004
Bai Y, Wang X, Zhao S, Ma C, Cui J, Zheng Y (2015) Sulforaphane protects against cardiovascular disease via Nrf2 activation. Oxidative Med Cell Longev 2015:407580. doi:10.1155/2015/407580
de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM (2015) The effects of baicalein and baicalin on mitochondrial function and dynamics: a review. Pharmacol Res 100:296–308. doi:10.1016/j.phrs.2015.08.021
de Oliveira MR (2016) Phloretin-induced cytoprotective effects on mammalian cells: a mechanistic view and future directions. Biofactors 42:13–40. doi:10.1002/biof.1256
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016) Resveratrol and the mitochondria: from triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860:727–745. doi:10.1016/j.bbagen.2016.01.017
de Oliveira MR (2016) The effects of ellagic acid upon brain cells: a mechanistic view and future directions. Neurochem Res 41:1219–1228. doi:10.1007/s11064-016-1853-9
Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM (2016) Epigallocatechin gallate and mitochondria—a story of life and death. Pharmacol Res 104:70–85. doi:10.1016/j.phrs.2015.12.027
Gibbs PE, Maines MD (2007) Biliverdin inhibits activation of NF-kappaB: reversal of inhibition by human biliverdin reductase. Int J Cancer 121:2567–2574
Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S (2010) Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol 80:1895–1903. doi:10.1016/j.bcp.2010.07.014
Gullotta F, di Masi A, Coletta M, Ascenzi P (2012) CO metabolism, sensing, and signaling. Biofactors 38:1–13
O’Brien L, Hosick PA, John K, Stec DE, Hinds TD Jr (2015) Biliverdin reductase isozymes in metabolism. Trends Endocrinol Metab 26:212–220. doi:10.1016/j.tem.2015.02.001
Kim HP, Ryter SW, Choi AM (2006) CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol 46:411–449
Desmard M, Boczkowski J, Poderoso J, Motterlini R (2007) Mitochondrial and cellular heme-dependent proteins as targets for the bioactive function of the heme oxygenase/carbon monoxide system. Antioxid Redox Signal 9:2139–2155
Lukiw WJ, Bazan NG (2000) Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem Res 25:1173–1184
Mattson MP (2005) NF-kappaB in the survival and plasticity of neurons. Neurochem Res 30:883–893
Lee CH, Jeon YT, Kim SH, Song YS (2007) NF-kappaB as a potential molecular target for cancer therapy. Biofactors 29:19–35
Shih RH, Wang CY, Yang CM (2015) NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 8:77. doi:10.3389/fnmol.2015.00077
Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN (2008) Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–1489. doi:10.1016/j.bcp.2008.07.017
Pan H, Wang H, Wang X, Zhu L, Mao L (2012) The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat Inflamm 2012:217580. doi:10.1155/2012/217580
Cuadrado A, Martín-Moldes Z, Ye J, Lastres-Becker I (2014) Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem 289:15244–15258. doi:10.1074/jbc.M113.540633
Ramyaa P, Krishnaswamy R, Padma VV (2014) Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim Biophys Acta 1840:681–692. doi:10.1016/j.bbagen.2013.10.024
Li W, Suwanwela NC, Patumraj S (2016) Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res 106:117–127. doi:10.1016/j.mvr.2015.12.008
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Hoesel B, Schmid JA (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12:86. doi:10.1186/1476-4598-12-86
Kay E, Scotland RS, Whiteford JR (2014) Toll-like receptors: role in inflammation and therapeutic potential. Biofactors 40:284–294
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653
Arias-Salvatierra D, Silbergeld EK, Acosta-Saavedra LC, Calderon-Aranda ES (2011) Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by lipopolysaccharide. Cell Signal 23:425–435. doi:10.1016/j.cellsig.2010.10.017
Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, Bazer FW, Wu G (2013) Nitric oxide and energy metabolism in mammals. Biofactors 39:383–391
Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J et al (2004) The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem 385:1–10
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Gama CS, Bosco SM (2016) Carnosic acid protects mitochondria of human neuroblastoma SH-SY5Y cells exposed to paraquat through activation of the Nrf2/HO-1Axis. Mol Neurobiol. doi:10.1007/s12035-016-0100-3
Yang W, Tiffany-Castiglioni E, Lee MY, Son IH (2010) Paraquat induces cyclooxygenase-2 (COX-2) implicated toxicity in human neuroblastoma SH-SY5Y cells. Toxicol Lett 199:239–246. doi:10.1016/j.toxlet.2010.09.005
Liu MW, Su MX, Zhang W, Wang YQ, Chen M, Wang L, Qian CY (2014) Protective effect of Xuebijing injection on paraquat-induced pulmonary injury via down-regulating the expression of p38 MAPK in rats. BMC Complement Altern Med 14:498. doi:10.1186/1472-6882-14-498
Han J, Ma D, Zhang M, Yang X, Tan D (2015) Natural antioxidant betanin protects rats from paraquat-induced acute lung injury interstitial pneumonia. Biomed Res Int 2015:608174. doi:10.1155/2015/608174
Amirshahrokhi K, Khalili AR (2016) Carvedilol attenuates paraquat-induced lung injury by inhibition of proinflammatory cytokines, chemokine MCP-1, NF-κB activation and oxidative stress mediators. Cytokine 88:144–153. doi:10.1016/j.cyto.2016.09.004
Miller RL, James-Kracke M, Sun GY, Sun AY (2009) Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34:55–65. doi:10.1007/s11064-008-9656-2
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62:803–819. doi:10.1016/j.neuint.2012.12.016
Anderson G, Maes M (2014) Neurodegeneration in Parkinson’s disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Mol Neurobiol 49:771–783. doi:10.1007/s12035-013-8554-z
Kumar A, Leinisch F, Kadiiska MB, Corbett J, Mason RP (2016) Formation and implications of alpha-synuclein radical in Maneb- and paraquat-induced models of Parkinson’s disease. Mol Neurobiol 53:2983–2994. doi:10.1007/s12035-015-9179-1
Baltazar MT, Dinis-Oliveira RJ, de Lourdes BM, Tsatsakis AM, Duarte JA, Carvalho F (2014) Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases—a mechanistic approach. Toxicol Lett 230:85–103. doi:10.1016/j.toxlet.2014.01.039
Khalighi Z, Rahmani A, Cheraghi J, Ahmadi MR, Soleimannejad K, Asadollahi R, Asadollahi K (2016) Perfluorocarbon attenuates inflammatory cytokines, oxidative stress and histopathologic changes in paraquat-induced acute lung injury in rats. Environ Toxicol Pharmacol 42:9–15. doi:10.1016/j.etap.2015.12.002
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: role for PI3K/Akt/Nrf2 pathway. Toxicol in Vitro 32:41–54. doi:10.1016/j.tiv.2015.12.005
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. doi:10.1016/j.cbi.2015.11.003
de Oliveira MR, Peres A, Gama CS, Bosco SM (2016) Pinocembrin provides mitochondrial protection by the activation of the Erk1/2-Nrf2 signaling pathway in SH-SY5Y neuroblastoma cells exposed to paraquat. Mol Neurobiol. doi:10.1007/s12035-016-0135-5
de Oliveira MR, da Rocha RF, Stertz L, Fries GR, de Oliveira DL, Kapczinski F, Moreira JC (2011) Total and mitochondrial nitrosative stress, decreased brain-derived neurotrophic factor (BDNF) levels and glutamate uptake, and evidence of endoplasmic reticulum stress in the hippocampus of vitamin A-treated rats. Neurochem Res 36:506–517. doi:10.1007/s11064-010-0372-3
de Oliveira MR, Lorenzi R, Schnorr CE, Morrone M, Moreira JC (2011) Increased 3-nitrotyrosine levels in mitochondrial membranes and impaired respiratory chain activity in brain regions of adult female rats submitted to daily vitamin a supplementation for 2 months. Brain Res Bull 86:246–253. doi:10.1016/j.brainresbull.2011.08.006
de Oliveira MR, da Rocha RF, Pasquali MA, Moreira JC (2012) The effects of vitamin a supplementation for 3 months on adult rat nigrostriatal axis: increased monoamine oxidase enzyme activity, mitochondrial redox dysfunction, increased β-amyloid(1-40) peptide and TNF-α contents, and susceptibility of mitochondria to an in vitro H2O2 challenge. Brain Res Bull 87:432–444. doi:10.1016/j.brainresbull.2012.01.005
de Oliveira MR, Schuck PF, Bosco SM (2016) Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Mol Neurobiol. doi:10.1007/s12035-016-0009-x
Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J et al (2008) Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem 104:1116–1131
Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, Maes M (2014) The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol 50:1059–1084. doi:10.1007/s12035-014-8705-x
Denzer I, Münch G, Friedland K (2016) Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res 103:80–94. doi:10.1016/j.phrs.2015.11.019
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34:532–549. doi:10.1016/j.biotechadv.2015.12.014
Oh J, Yu T, Choi SJ, Yang Y, Baek HS, An SA, Kwon LK et al (2012) Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediat Inflamm 2012:781375. doi:10.1155/2012/781375
Schwager J, Richard N, Fowler A, Seifert N, Raederstorff D (2016) Carnosol and related substances modulate chemokine and cytokine production in macrophages and chondrocytes. Molecules 21:465. doi:10.3390/molecules21040465
Heinecke LF, Grzanna MW, Au AY, Mochal CA, Rashmir-Raven A, Frondoza CG (2010) Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthr Cartil 18:220–227. doi:10.1016/j.joca.2009.08.015
Luo C, Urgard E, Vooder T, Metspalu A (2011) The role of COX-2 and Nrf2/ARE in anti-inflammation and antioxidative stress: aging and anti-aging. Med Hypotheses 77:174–178. doi:10.1016/j.mehy.2011.04.002
Nørregaard R, Kwon TH, Frøkiær J (2015) Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res Clin Pract 34:194–200. doi:10.1016/j.krcp.2015.10.004
Qin WS, Deng YH, Cui FC (2016) Sulforaphane protects against acrolein-induced oxidative stress and inflammatory responses: modulation of Nrf-2 and COX-2 expression. Arch Med Sci 12:871–880. doi:10.5114/aoms.2016.59919
Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D (2016) ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Med Cell Longev 2016:3565127. doi:10.1155/2016/3565127
Morris G, Berk M, Klein H, Walder K, Galecki P, Maes M (2016) Nitrosative stress, hypernitrosylation, and autoimmune responses to nitrosylated proteins: new pathways in neuroprogressive disorders including depression and chronic fatigue syndrome. Mol Neurobiol. doi:10.1007/s12035-016-9975-2
Rehman MU, Tahir M, Khan AQ, Khan R, Lateef A, Oday-O-Hamiza QW, Ali F, Sultana S (2013) Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett 216:146–158. doi:10.1016/j.toxlet.2012.11.013
Sharma N, Nehru B (2015) Characterization of the lipopolysaccharide induced model of Parkinson’s disease: role of oxidative stress and neuroinflammation. Neurochem Int 87:92–105. doi:10.1016/j.neuint.2015.06.004
Bhattacharjee N, Borah A (2016) Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson’s disease. Neurochem Int 101:48–55. doi:10.1016/j.neuint.2016.10.001
Niranjan R (2014) The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol Neurobiol 49:28–38. doi:10.1007/s12035-013-8483-x
Ljubisavljevic S, Stojanovic I (2015) Neuroinflammation and demyelination from the point of nitrosative stress as a new target for neuroprotection. Rev Neurosci 26:49–73. doi:10.1515/revneuro-2014-0060
Ljubisavljevic S (2016) Oxidative stress and neurobiology of demyelination. Mol Neurobiol 53:744–758. doi:10.1007/s12035-014-9041-x
Hulsmans M, Holvoet P (2010) The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med 14:70–78. doi:10.1111/j.1582-4934.2009.00978.x
Wu JJ, Zhu YT, Hu YM (2016) Mechanism of feedback regulation of neutrophil inflammation in Henoch-Schönlein purpura. Eur Rev Med Pharmacol Sci 20:4277–4285
Li H, Sun JJ, Chen GY, Wang WW, Xie ZT, Tang GF, Wei SD (2016) Carnosic acid nanoparticles suppress liver ischemia/reperfusion injury by inhibition of ROS, caspases and NF-κB signaling pathway in mice. Biomed Pharmacother 82:237–246. doi:10.1016/j.biopha.2016.04.064
Foresti R, Bains SK, Pitchumony TS, de Castro Brás LE, Drago F, Dubois-Randé JL, Bucolo C, Motterlini R (2013) Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res 76:132–148. doi:10.1016/j.phrs.2013.07.010
Tsai CW, Liu KL, Lin YR, Kuo WC (2014) The mechanisms of carnosic acid attenuates tumor necrosis factor-α-mediated inflammation and insulin resistance in 3 T3-L1 adipocytes. Mol Nutr Food Res 58:654–664. doi:10.1002/mnfr.201300356
Acknowledgements
ICCS and CRF received a MCTI/CNPq/Universal 14/2014 fellow. This work was supported by CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., de Souza, I.C.C. & Fürstenau, C.R. Carnosic Acid Induces Anti-Inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Involving a Crosstalk Between the Nrf2/HO-1 Axis and NF-κB. Mol Neurobiol 55, 890–897 (2018). https://doi.org/10.1007/s12035-017-0389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0389-6