Abstract
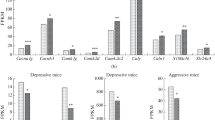
Chronic stress is a risk factor for major depression. Social defeat stress is a well-validated murine model of depression. However, little is known about the gene activity dynamics during the development of a depression-like state. We analyzed the effects of social defeat stress of varying duration (10 and 30 days) on the behavioral patterns and prefrontal-cortex transcriptome of C57BL/6 mice. The 10-day exposure to social defeat stress resulted in a high level of social avoidance with no signs of depression-associated behavior. Most animals exposed to 30 days of social defeat stress demonstrated clear hallmarks of depression, including a higher level of social avoidance, increased immobility in the forced swimming test, and anhedonic behavior. The monitoring of transcriptome changes revealed widespread alterations in gene expression on the 10th day. Surprisingly, the expression of only a few genes were affected by the 30th day of stress, apparently due to a reversal of the majority of the early stress-induced changes to the original basal state. Moreover, we have found that glucocorticoid-sensitive genes are clearly stimulated targets on the 10th day of stress, but these genes stop responding to the elevated corticosterone level by the 30th day of stress. The majority of genes altered by the 30-day stress were downregulated, with the most relevant ones participating in chromatin modifications and neuroplasticity (e.g., guanine nucleotide exchange factors of the Rho-family of GTPases). Very different molecular responses occur during short-term and long-term social stress in mice. The early-stress response is associated with social avoidance and with upregulation and downregulation of many genes, including those related to signal transduction and cell adhesion pathways. Downregulation of a few genes, in particular, genes for histone-modifying methyltransferases, is a signature response to prolonged stress that induces symptoms of depression. Altogether, our data show that the development of depression under social stress conditions is correlated with suppression of the overactive molecular response to induced stress, involving gene regulatory resistance to glucocorticoid molecules, potentially via a chromatin remodeling mechanism.






Similar content being viewed by others
References
Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA (2013) Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 10(11):e1001547. doi:10.1371/journal.pmed.1001547
Challis C, Berton O (2015) Top-down control of serotonin systems by the prefrontal cortex: a path towards restored socioemotional function in depression. ACS Chem Neurosci 6(7):1040–1054. doi:10.1021/acschemneuro.5b00007
Covington HE 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E et al (2010) Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci 30(48):16082–16090. doi:10.1523/JNEUROSCI.1731-10.2010
Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M et al (2014) Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci 34(11):3878–3887. doi:10.1523/JNEUROSCI.1787-13.2014
Lehmann ML, Herkenham M (2011) Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci 31(16):6159–6173. doi:10.1523/JNEUROSCI.0577-11.2011
Albert PR, Vahid-Ansari F, Luckhart C (2014) Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 8:199. doi:10.3389/fnbeh.2014.00199
Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ (2007) GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32(2):471–482. doi:10.1038/sj.npp.1301234
Hamani C, Mayberg H, Snyder B, Giacobbe P, Kennedy S, Lozano AM (2009) Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg 111(6):1209–1215. doi:10.3171/2008.10.JNS08763
Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P (2014) Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord 159:31–38. doi:10.1016/j.jad.2014.02.016
Holtzheimer PE, Mayberg HS (2011) Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci 34:289–307. doi:10.1146/annurev-neuro-061010-113638
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM et al (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762):864–868. doi:10.1126/science.1120972
Kudryavtseva NN, Avgustinovich DF (1998) Behavioral and physiological markers of experimental depression induced by social conflicts (DISC). Aggress Behav 24:271–286. doi:10.1002/(SICI)1098-2337(1998)24:4<271::AID-AB3>3.0.CO;2-M
Venzala E, Garcia-Garcia AL, Elizalde N, Delagrange P, Tordera RM (2012) Chronic social defeat stress model: behavioral features, antidepressant action, and interaction with biological risk factors. Psychopharmacology 224(2):313–325. doi:10.1007/s00213-012-2754-5
Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA (1991) Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav 38(2):315–320
Bartolomucci A, Leopardi R (2009) Stress and depression: preclinical research and clinical implications. PLoS One 4(1):e4265. doi:10.1371/journal.pone.0004265
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A et al (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131(2):391–404. doi:10.1016/j.cell.2007.09.018
Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q et al (2013) Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry 73(1):7–14. doi:10.1016/j.biopsych.2012.06.006
Stankiewicz AM, Goscik J, Majewska A, Swiergiel AH, Juszczak GR (2015) The effect of acute and chronic social stress on the hippocampal transcriptome in mice. PLoS One 10(11):e0142195. doi:10.1371/journal.pone.0142195
Stankiewicz AM, Goscik J, Swiergiel AH, Majewska A, Wieczorek M, Juszczak GR, Lisowski P (2014) Social stress increases expression of hemoglobin genes in mouse prefrontal cortex. BMC Neurosci 15(1):130. doi:10.1186/s12868-014-0130-6
Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, Huang X, Schluter OM et al (2016) Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron 90(5):969–983. doi:10.1016/j.neuron.2016.04.015
Tordera RM, Garcia-Garcia AL, Elizalde N, Segura V, Aso E, Venzala E, Ramirez MJ, Del Rio J (2011) Chronic stress and impaired glutamate function elicit a depressive-like phenotype and common changes in gene expression in the mouse frontal cortex. Eur Neuropsychopharmacol 21(1):23–32. doi:10.1016/j.euroneuro.2010.06.016
Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, Scobie K, Bagot R et al (2014) Beta-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 516(7529):51–55. doi:10.1038/nature13976
Bondar NP, Kovalenko IL, Avgustinovich DF, Smagin DA, Kudryavtseva NN (2009) Anhedonia in the shadow of chronic social defeat stress, or when the experimental context matters. The Open Behavioral Science Journal 3:17–27. doi:10.2174/1874230000903010017
Avgustinovich DF, Kovalenko IL, Kudryavtseva NN (2005) A model of anxious depression: Persistence of behavioral pathology. Neurosci Behav Physiol 35(9):917–924. doi:10.1007/s11055-005-0146-6
Kudryavtseva NN, Bondar NP, Boyarskikh UA, Filipenko ML (2010) Snca and Bdnf gene expression in the VTA and raphe nuclei of midbrain in chronically victorious and defeated male mice. PLoS One 5(11):e14089. doi:10.1371/journal.pone.0014089
Boyarskikh UA, Bondar NP, Filipenko ML, Kudryavtseva NN (2013) Downregulation of serotonergic gene expression in the raphe nuclei of the midbrain under chronic social defeat stress in male mice. Mol Neurobiol 48(1):13–21. doi:10.1007/s12035-013-8413-y
Merkulov VM, Merkulova TI, Bondar NP (2017) Mechanisms of brain glucocorticoid resistance in stress-induced psychopathologies. Biochemistry (Mosc) 82(3):351–365. doi:10.1134/S0006297917030142
Pariante CM, Lightman SL (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31(9):464–468. doi:10.1016/j.tins.2008.06.006
Cattaneo A, Riva MA (2015) Stress-induced mechanisms in mental illness: a role for glucocorticoid signalling. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2015.07.021
Wu X, Wu J, Xia S, Li B, Dong J (2013) Icaritin opposes the development of social aversion after defeat stress via increases of GR mRNA and BDNF mRNA in mice. Behav Brain Res 256:602–608. doi:10.1016/j.bbr.2013.09.034
Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M (2013) Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci 33(7):2961–2972. doi:10.1523/JNEUROSCI.3878-12.2013
Koch CE, Bartlang MS, Kiehn JT, Lucke L, Naujokat N, Helfrich-Forster C, Reber SO, Oster H (2016) Time-of-day-dependent adaptation of the HPA axis to predictable social defeat stress. J Endocrinol 231(3):209–221. doi:10.1530/JOE-16-0163
Brain PF, McAllister KH, Walmsley SV (1989) Drug effects on social behaviour: methods in ethopharmacology. In: Boulton AA, Baker GB, Greenshaw AJ (eds) Neuromethods. psychopharmacology. The Humana Press Inc, Clifton, NJ, pp. 687–739
Kudryavtseva NN (2003) Use of the “partition” test in behavioral and pharmacological experiments. Neurosci Behav Physiol 33(5):461–471. doi:10.1023/A:1023411217051
Castagne V, Porsolt RD, Moser P (2009) Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur J Pharmacol 616(1–3):128–133. doi:10.1016/j.ejphar.2009.06.018
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229(2):327–336
Avgustinovich DF, Kovalenko IL, Bondar NP (2005) Choice of “control” in experimental researches of animal social interactions in mice. Ross Fiziol Zh Im I M Sechenova 91(12):1454–1468
Kudryavtseva NN (2011) Sensory contact model: protocol, control, applications. Nova Science Publishers, Inc, New York
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol. doi:10.1111/2041-210X.12584
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. doi:10.1093/bioinformatics/btu170
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14(4):R36. doi:10.1186/gb-2013-14-4-r36
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550. doi:10.1186/s13059-014-0550-8
Wang J, Duncan D, Shi Z, Zhang B (2013) WEB-based GEne SeT AnaLysis toolkit (WebGestalt): Update 2013. Nucleic Acids Res 41 (web server issue):W77–83. doi:10.1093/nar/gkt439
Zhang B, Kirov S, Snoddy J (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33 (web server issue):W741–748. doi:10.1093/nar/gki475
Carter BS, Meng F, Thompson RC (2012) Glucocorticoid treatment of astrocytes results in temporally dynamic transcriptome regulation and astrocyte-enriched mRNA changes in vitro. Physiol Genomics 44(24):1188–1200. doi:10.1152/physiolgenomics.00097.2012
John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA (2011) Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet 43(3):264–268. doi:10.1038/ng.759
Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang JC (2012) Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci U S A 109(28):11160–11165. doi:10.1073/pnas.1111334109
Yu CY, Mayba O, Lee JV, Tran J, Harris C, Speed TP, Wang JC (2010) Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One 5(12):e15188. doi:10.1371/journal.pone.0015188
Lee HY, Gao X, Barrasa MI, Li H, Elmes RR, Peters LL, Lodish HF (2015) PPAR-alpha and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature 522(7557):474–477. doi:10.1038/nature14326
Grontved L, John S, Baek S, Liu Y, Buckley JR, Vinson C, Aguilera G, Hager GL (2013) C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements. EMBO J 32(11):1568–1583. doi:10.1038/emboj.2013.106
Polman JA, Welten JE, Bosch DS, de Jonge RT, Balog J, van der Maarel SM, de Kloet ER, Datson NA (2012) A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci 13:118. doi:10.1186/1471-2202-13-118
Polman JA, de Kloet ER, Datson NA (2013) Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome. Endocrinology 154(5):1832–1844. doi:10.1210/en.2012-2187
Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13(10):1161–1169. doi:10.1038/nn.2647
Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P (2004) Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology 29(7):899–910. doi:10.1016/j.psyneuen.2003.08.003
Duque A, Vinader-Caerols C, Monleon S (2017) Indomethacin counteracts the effects of chronic social defeat stress on emotional but not recognition memory in mice. PLoS One 12(3):e0173182. doi:10.1371/journal.pone.0173182
Veeraiah P, Noronha JM, Maitra S, Bagga P, Khandelwal N, Chakravarty S, Kumar A, Patel AB (2014) Dysfunctional glutamatergic and gamma-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol Psychiatry 76(3):231–238. doi:10.1016/j.biopsych.2013.09.024
Kanarik M, Alttoa A, Matrov D, Koiv K, Sharp T, Panksepp J, Harro J (2011) Brain responses to chronic social defeat stress: effects on regional oxidative metabolism as a function of a hedonic trait, and gene expression in susceptible and resilient rats. Eur Neuropsychopharmacol 21(1):92–107. doi:10.1016/j.euroneuro.2010.06.015
Pariante CM, Miller AH (2001) Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49(5):391–404. doi:10.1016/S0006-3223(00)01088-X
Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23(5):477–501. doi:10.1016/S0893-133X(00)00159-7
Menke A, Arloth J, Putz B, Weber P, Klengel T, Mehta D, Gonik M, Rex-Haffner M et al (2012) Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology 37(6):1455–1464. doi:10.1038/npp.2011.331
Fuchsl AM, Reber SO (2016) Chronic psychosocial stress and negative feedback inhibition: enhanced hippocampal glucocorticoid signaling despite lower cytoplasmic GR expression. PLoS One 11(4):e0153164. doi:10.1371/journal.pone.0153164
Merkulov VM, Merkulova TI (2012) Glucocorticoid receptor isoforms generated by alternative splicing and alternative translation initiation. Russ J Genetics 2(3):205–213
Oakley RH, Cidlowski JA (2011) Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem 286(5):3177–3184. doi:10.1074/jbc.R110.179325
Akbarian S, Huang HS (2009) Epigenetic regulation in human brain-focus on histone lysine methylation. Biol Psychiatry 65(3):198–203. doi:10.1016/j.biopsych.2008.08.015
Shen E, Shulha H, Weng Z, Akbarian S (2014) Regulation of histone H3K4 methylation in brain development and disease. Philos Trans R Soc Lond B Biol Sci 369(1652). doi:10.1098/rstb.2013.0514
Bonneh-Barkay D, Wiley CA (2009) Brain extracellular matrix in neurodegeneration. Brain Pathol 19(4):573–585. doi:10.1111/j.1750-3639.2008.00195.x
Kerrisk ME, Cingolani LA, Koleske AJ (2014) ECM receptors in neuronal structure, synaptic plasticity, and behavior. Prog Brain Res 214:101–131. doi:10.1016/B978-0-444-63486-3.00005-0
Li XH, Chen JX, Yue GX, Liu YY, Zhao X, Guo XL, Liu Q, Jiang YM et al (2013) Gene expression profile of the hippocampus of rats subjected to chronic immobilization stress. PLoS One 8(3):e57621. doi:10.1371/journal.pone.0057621
Barber M, Di Meglio T, Andrews WD, Hernandez-Miranda LR, Murakami F, Chedotal A, Parnavelas JG (2009) The role of Robo3 in the development of cortical interneurons. Cereb Cortex 19(Suppl 1):i22–i31. doi:10.1093/cercor/bhp041
Dickinson RE, Duncan WC (2010) The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction 139(4):697–704. doi:10.1530/REP-10-0017
Aasheim HC, Patzke S, Hjorthaug HS, Finne EF (2005) Characterization of a novel Eph receptor tyrosine kinase, EphA10, expressed in testis. Biochim Biophys Acta 1723(1–3):1–7. doi:10.1016/j.bbagen.2005.01.011
Bouvier G, Bidoret C, Casado M, Paoletti P (2015) Presynaptic NMDA receptors: roles and rules. Neuroscience 311:322–340. doi:10.1016/j.neuroscience.2015.10.033
Sandi C (2004) Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci 5(12):917–930. doi:10.1038/nrn1555
Dityatev A, Schachner M, Sonderegger P (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11(11):735–746. doi:10.1038/nrn2898
Acknowledgments
This work was supported by grants #14-04-01707 from the Russian Foundation for Basic Research (behavioral study), a grant from the Government of the Russian Federation #14.B25.31.0033 (HiSeq Illumina sequencing), a grant from the Russian Science Foundation #14-44-00077 (analysis of ChIP-seq datasets), and a grant from the Russian Science Foundation #16-15-10131 (analysis of RNA-seq data and dataset on dexamethasone-affected genes).
All behavioral experiments were conducted in the Laboratory of Experimental Models of Neurodegenerative Processes at the Institute of Physiology and Basic Medicine, Novosibirsk, Russia. We would like to thank Pavlov K.S. for technical assistance with the behavioral testing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Bondar, N., Bryzgalov, L., Ershov, N. et al. Molecular Adaptations to Social Defeat Stress and Induced Depression in Mice. Mol Neurobiol 55, 3394–3407 (2018). https://doi.org/10.1007/s12035-017-0586-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0586-3