Abstract
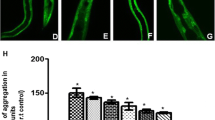
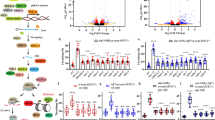
Circular RNAs (circRNAs) are peculiar non-coding RNA molecules which are known to be present across taxa. Considering the body of evidence that establishes critical functions of non-coding RNA molecules, we endeavored to study circRNAs in the context of Parkinson’s disease (PD). Employing transgenic C. elegans model of PD, we used RNase R-mediated cleavage of linear RNA followed by divergent primer-based amplifications towards identifying circzip-2, a novel circRNA molecule. We went on to sequence circzip-2 which is synthesized from functionally important gene zip-2. Studying RNAi-induced knockdown conditions of zip-2, we observed a reduced aggregation of α-synuclein protein along with an enhanced lifespan of the worms. We further carried out transcriptome analysis of zip-2 silenced worms, which suggested that zip-2 might be functioning via Daf-16 pathway. Further interaction studies revealed that circzip-2 possibly sponges microRNA molecule miR-60 towards asserting an important role in various processes associated with PD.




Similar content being viewed by others
References
Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO (2012) Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 7(2):e30733. https://doi.org/10.1371/journal.pone.0030733
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441):333–338. https://doi.org/10.1038/nature11928
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388. https://doi.org/10.1038/nature11993
Wang PL, Bao Y, Yee MC, Barrett SP, Hogan GJ, Olsen MN, Dinneny JR, Brown PO et al (2014) Circular RNA is expressed across the eukaryotic tree of life. PLoS One 9(6):e90859. https://doi.org/10.1371/journal.pone.0090859
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK (1976) Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A 73(11):3852–3856. https://doi.org/10.1073/pnas.73.11.3852
Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B (1991) Scrambled exons. Cell 64(3):607–613. https://doi.org/10.1016/0092-8674(91)90244-S
Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R (1993) Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73(5):1019–1030. https://doi.org/10.1016/0092-8674(93)90279-Y
Bailleul B (1996) During in vivo maturation of eukaryotic nuclear mRNA, splicing yields excised exon circles. Nucleic Acids Res 24(6):1015–1019. https://doi.org/10.1093/nar/24.6.1015
Caldas C, So CW, MacGregor A, Ford AM, McDonald B, Chan LC, Wiedemann LM (1998) Exon scrambling of MLL transcripts occur commonly and mimic partial genomic duplication of the gene. Gene 208(2):167–176. https://doi.org/10.1016/S0378-1119(97)00640-9
Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE (2010) Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 6(12):e1001233. https://doi.org/10.1371/journal.pgen.1001233
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19(2):141–157. https://doi.org/10.1261/rna.035667.112
Suzuki H, Zuo Y, Wang J, Zhang MQ, Malhotra A, Mayeda A (2006) Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res 34(8):e63. https://doi.org/10.1093/nar/gkl151
Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM (2009) Repression of α-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A 106(31):13052–13057. https://doi.org/10.1073/pnas.0906277106
Saydam O, Senol O, Wurdinger T, Mizrak A, Ozdener GB, Stemmer-Rachamimov AO, Yi M, Stephens RM et al (2011) miRNA-7 attenuation in Schwannoma tumors stimulates growth by upregulating three oncogenic signaling pathways. Cancer Res 71(3):852–861. https://doi.org/10.1158/0008-5472.CAN-10-1219
Fang Y, Xue JL, Shen Q, Chen J, Tian L (2012) MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 55(6):1852–1862. https://doi.org/10.1002/hep.25576
Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ (2009) Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem 284(9):5731–5741. https://doi.org/10.1074/jbc.M804280200
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S et al (2014) circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56(1):55–66. https://doi.org/10.1016/j.molcel.2014.08.019
Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443(7113):780–786. https://doi.org/10.1038/nature05291
Koller WC, Tse W (2004) Unmet medical needs in Parkinson’s disease. Neurology 62(1 Suppl 1):S1–S8. https://doi.org/10.1212/WNL.62.1_suppl_1.S1
Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR et al (2014) Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep 9(5):1966–1980. https://doi.org/10.1016/j.celrep.2014.10.062
Vincent HA, Deutscher MP (2006) Substrate recognition and catalysis by the exoribonuclease RNase R. J Biol Chem 281(40):29769–29775. https://doi.org/10.1074/jbc.M606744200
Pukkila-Worley R, Feinbaum R, Kirienko NV, Larkins-Ford J, Conery AL, Ausubel FM (2012) Stimulation of host immune defenses by a small molecule protects C. elegans from bacterial infection. PLoS Genet 8(6):e1002733. https://doi.org/10.1371/journal.pgen.1002733
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74. https://doi.org/10.2174/157015909787602823
Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389(6654):994–999. https://doi.org/10.1038/40194
Webb AE, Kundaje A, Brunet A (2016) Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 15(4):673–685. https://doi.org/10.1111/acel.12479
Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C et al (2010) The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38(Web Server issue):W214–W220. https://doi.org/10.1093/nar/gkq537
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T (1995) The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14(2):467–475. https://doi.org/10.1016/0896-6273(95)90302-X
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) α-synuclein in Lewy bodies. Nature 388(6645):839–840. https://doi.org/10.1038/42166
Kovacs KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR (2003) CCAAT/enhancer-binding protein family members recruit the coactivator CREB-binding protein and trigger its phosphorylation. J Biol Chem 278(38):36959–36965. https://doi.org/10.1074/jbc.M303147200
Kim S, Cheon HS, Kim SY, Juhnn YS, Kim YY (2013) Cadmium induces neuronal cell death through reactive oxygen species activated by GADD153. BMC Cell Biol 14(1):4. https://doi.org/10.1186/1471-2121-14-4
Kazkayasi I, Burul-Bozkurt N, Onder S, Kelicen-Ugur P, Pekiner C (2013) Effects of experimental diabetes on C/EBP proteins in rat hippocampus, sciatic nerve and ganglia. Cell Mol Neurobiol 33(4):559–567. https://doi.org/10.1007/s10571-013-9924-9
Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M (2010) Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int 10(Suppl 1):S148–S157. https://doi.org/10.1111/j.1447-0594.2010.00588.x
Robert I, Sutter A, Quirin-Stricker C (2002) Synergistic activation of the human choline acetyltransferase gene by c-Myb and C/EBPbeta. Brain Res Mol Brain Res 106(1–2):124–135. https://doi.org/10.1016/S0169-328X(02)00419-9
Haque R, Nazir A (2014) SMAD transcription factor, Sma-9, attunes TGF-beta signaling cascade towards modulating amyloid beta aggregation and associated outcome in transgenic C. elegans. Mol Neurobiol 53(1):109–119. https://doi.org/10.1007/s12035-014-8988-y
Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC et al (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136(1):62–74. https://doi.org/10.1016/j.cell.2008.10.052
Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V (2008) The role of ATF-2 in oncogenesis. BioEssays 30(4):314–327. https://doi.org/10.1002/bies.20734
Hoare S, Copland JA, Wood TG, Jeng YJ, Izban MG, Soloff MS (1999) Identification of a GABP alpha/beta binding site involved in the induction of oxytocin receptor gene expression in human breast cells, potentiation by c-Fos/c-Jun. Endocrinology 140(5):2268–2279. https://doi.org/10.1210/endo.140.5.6710
Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N et al (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421(6920):231–237. https://doi.org/10.1038/nature01278
Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT (2010) Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol 8(5):e1000372. https://doi.org/10.1371/journal.pbio.1000372
Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A (2011) Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470(7334):404–408. https://doi.org/10.1038/nature09706
Chao CW, Chan DC, Kuo A, Leder P (1998) The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis. Mol Med 4(9):614–628
Barrett SP, Salzman J (2016) Circular RNAs: analysis, expression and potential functions. Development 143(11):1838–1847. https://doi.org/10.1242/dev.128074
Kato M, Kashem MA, Cheng C (2016) An intestinal microRNA modulates the homeostatic adaptation to chronic oxidative stress in C. elegans. Aging 8(9):1979–2005. https://doi.org/10.18632/aging.101029
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77(1):71–94
Stiernagle T (2006) Maintenance of C. elegans. WormBook 11:1–11
Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408(6810):325–330. https://doi.org/10.1038/35042517
Jadiya P, Nazir A (2012) Environmental toxicants as extrinsic epigenetic factors for parkinsonism: studies employing transgenic C. elegans model. CNS Neurol Disord Drug Targets 11(8):976–983
Walter L, Baruah A, Chang HW, Pace HM, Lee SS (2011) The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS Biol 9(6):e1001084. https://doi.org/10.1371/journal.pbio.1001084
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25(9):1105–1111. https://doi.org/10.1093/bioinformatics/btp120
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359. https://doi.org/10.1038/nmeth.1923
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ et al (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515. https://doi.org/10.1038/nbt.1621
Acknowledgements
We are thankful of Caenorhabditis Genetics Center (CGC), University of Minnesota, MN, USA, for providing the C. elegans strains. CSIR-CDRI communication number is 9614.
Funding
CGC is funded by the NIH National Center for Research Resources (NCRR). LK is supported by Senior Research Fellowship from Council for Scientific and Industrial Research (CSIR), and S, HR, and SS are supported by Senior Research Fellowship from UGC. AN acknowledges financial support from CSIR and DST, Government of India.
Author information
Authors and Affiliations
Contributions
LK conducted the experiments, analyzed the data, and wrote the manuscript; S, JP, HR, and SS conducted the experiments. AN conceived the study, analyzed the data, wrote the manuscript, and provided reagents and support.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 805 kb)
Rights and permissions
About this article
Cite this article
Kumar, L., Shamsuzzama, Jadiya, P. et al. Functional Characterization of Novel Circular RNA Molecule, circzip-2 and Its Synthesizing Gene zip-2 in C. elegans Model of Parkinson’s Disease. Mol Neurobiol 55, 6914–6926 (2018). https://doi.org/10.1007/s12035-018-0903-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0903-5