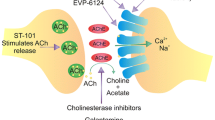
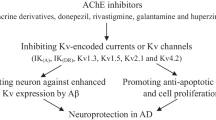
Abstract
Alzheimer’s disease (AD) is one of the most common forms of dementia among elder people, which is a progressive neurodegenerative disease that results from a chronic loss of cognitive activities. It has been observed that AD is multifactorial, hence diverse pharmacological targets that could be followed for the treatment of AD. The Food and Drug Administration has approved two types of medications for AD treatment such as cholinesterase inhibitors (ChEIs) and N-methyl-d-aspartic acid receptor (NMDAR) antagonists. Rivastigmine, donepezil, and galantamine are the ChEIs that have been approved to treat AD. On the other hand, memantine is the only non-competitive NMDAR antagonist approved in AD treatment. As compared with placebo, it has been revealed through clinical studies that many single-target therapies are unsuccessful to treat multifactorial Alzheimer’s symptoms or disease progression. Therefore, due to the complex nature of AD pathophysiology, diverse pharmacological targets can be hunted. In this article, based on the entwined link of acetylcholinesterase (AChE) and NMDAR, we represent several multifunctional compounds in the rational design of new potential AD medications. This review focus on the significance of privileged scaffolds in the generation of the multi-target lead compound for treating AD, investigating the idea and challenges of multi-target drug design. Furthermore, the most auspicious elementary units for designing as well as synthesizing hybrid drugs are demonstrated as pharmacological probes in the rational design of new potential AD therapeutics.























Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- MTDLs:
-
multi-target-directed ligands
- AChE:
-
acetylcholinesterase
- ACh:
-
acetylcholine
- NMDAR:
-
N-methyl-d-aspartic acid receptor
- Aβ:
-
amyloid beta
- NFTs:
-
neurofibrillary tangles
References
Sharma P, Sharma A, Fayaz F et al (2020) Biological signatures of Alzheimer’s disease. Curr Top Med Chem 20:770–781. https://doi.org/10.2174/1568026620666200228095553
Uddin MS, Kabir MT, Tewari D et al (2020) Revisiting the role of brain and peripheral Aβ in the pathogenesis of Alzheimer’s disease. J Neurol Sci 416:116974. https://doi.org/10.1016/j.jns.2020.116974
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. https://doi.org/10.15252/emmm.201606210
Uddin MS, Al Mamun A, Kabir MT et al (2019) Nootropic and anti-Alzheimer’s actions of medicinal plants: molecular insight into therapeutic potential to alleviate Alzheimer’s neuropathology. Mol Neurobiol 56:4925–4944. https://doi.org/10.1007/s12035-018-1420-2
Mufson EJ, Counts SE, Perez SE, Ginsberg SD (2008) Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother 8:1703–1718. https://doi.org/10.1586/14737175.8.11.1703
Uddin MS, Al Mamun A, Asaduzzaman M et al (2018) Spectrum of disease and prescription pattern for outpatients with neurological disorders: an empirical pilot study in Bangladesh. Ann Neurosci 25:25–37. https://doi.org/10.1159/000481812
Yan R, Vassar R (2014) Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol 13:319–329. https://doi.org/10.1016/S1474-4422(13)70276-X
Uddin MS, Al Mamun A, Rahman MA et al (2020) Emerging proof of protein misfolding and interactions in multifactorial Alzheimer’s disease. Curr Top Med Chem:20. https://doi.org/10.2174/1568026620666200601161703
Festa G, Mallamace F, Sancesario GM et al (2019) Aggregation states of Aβ1-40, Aβ1-42 and Aβp3-42 amyloid beta peptides: a SANS study. Int J Mol Sci 20. https://doi.org/10.3390/ijms20174126
Mamun A, Uddin M, Mathew B, Ashraf G (2020) Toxic tau: structural origins of tau aggregation in Alzheimer’s disease. Neural Regen Res 15:1417–1420. https://doi.org/10.4103/1673-5374.274329
Iqbal K, Alonso A d C, Chen S et al (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta Mol basis Dis 1739:198–210. https://doi.org/10.1016/j.bbadis.2004.09.008
Uddin MS, Mamun AA, Kabir MT et al (2018) Neurochemistry of neurochemicals: messengers of brain functions. J Intellect Disabil - Diagnosis Treat 5:137–151. https://doi.org/10.6000/2292-2598.2017.05.04.6
Parihar MS, Brewer GJ (2010) Amyloid-β as a modulator of synaptic plasticity. J Alzheimers Dis 22:741–763. https://doi.org/10.3233/JAD-2010-101020
Uddin MS, Kabir MT, Niaz K, Jeandet P, Clément C, Mathew B, Rauf A, Rengasamy KRR et al (2020) Molecular insight into the therapeutic promise of flavonoids against Alzheimer’s disease. Molecules 25:1267. https://doi.org/10.3390/MOLECULES25061267
Sheng M, Sabatini BL, Südhof TC (2012) Synapses and Alzheimer’s disease. Cold Spring Harb Perspect Biol 4:10. https://doi.org/10.1101/cshperspect.a005777
Maurer SV, Williams CL (2017) The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol 8:1489. https://doi.org/10.3389/fimmu.2017.01489
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115. https://doi.org/10.2174/1570159x13666150716165726
Lahiri D, Rogers J, Greig N, Sambamurti K (2004) Rationale for the development of cholinesterase inhibitors as anti-Alzheimer agents. Curr Pharm Des 10:3111–3119. https://doi.org/10.2174/1381612043383331
Herholz K (2008) Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur J Nucl Med Mol Imaging 35:25–29. https://doi.org/10.1007/s00259-007-0699-4
Uddin MS, Al Mamun A, Takeda S et al (2018) Analyzing the chance of developing dementia among geriatric people: a cross-sectional pilot study in Bangladesh. Psychogeriatrics. 19:87–94. https://doi.org/10.1111/psyg.12368
Wang R, Reddy PH (2017) Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis 57:1041–1048. https://doi.org/10.3233/JAD-160763
Lipton S (2005) The molecular basis of Memantine action in Alzheimers disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res 2:155–165. https://doi.org/10.2174/1567205053585846
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276. https://doi.org/10.1002/neu.480230915
Koh JY, Choi DW (1991) Selective blockade of non-NMDA receptors does not block rapidly triggered glutamate-induced neuronal death. Brain Res 548:318–321. https://doi.org/10.1016/0006-8993(91)91140-v
Tymianski M, Charlton MP, Carlen PL, Tator CH (1993) Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci 13:2085–2104
Mizuno S, Iijima R, Ogishima S et al (2012) AlzPathway: a comprehensive map of signaling pathways of Alzheimer’s disease. BMC Syst Biol 6:52. https://doi.org/10.1186/1752-0509-6-52
Uddin MS, Kabir MT, Tewari D et al (2020) Emerging therapeutic promise of ketogenic diet to attenuate neuropathological alterations in Alzheimer’s disease. Mol Neurobiol:1–17. https://doi.org/10.1007/s12035-020-02065-3
Yiannopoulou KG, Papageorgiou SG (2013) Current and future treatments for Alzheimer’s disease. Ther Adv Neurol Disord 6:19–33. https://doi.org/10.1177/1756285612461679
Umar T, Hoda N (2018) Alzheimer’s disease: a systemic review of substantial therapeutic targets and the leading multi-functional molecules. Curr Top Med Chem 17:3370–3389. https://doi.org/10.2174/1568026618666180112161024
Oset-Gasque MJ, Marco-Contelles J (2018) Alzheimer’s disease, the “one-molecule, one-target” paradigm, and the multitarget directed ligand approach. ACS Chem Neurosci 9:401–403. https://doi.org/10.1021/acschemneuro.8b00069
Agis-Torres A, Sölhuber M, Fernandez M, Sanchez-Montero JM (2014) Multi-target-directed ligands and other therapeutic strategies in the search of a real solution for Alzheimer’s disease. Curr Neuropharmacol 12:2–36. https://doi.org/10.2174/1570159X113116660047
Wang T, Liu XH, Guan J et al (2019) Advancement of multi-target drug discoveries and promising applications in the field of Alzheimer’s disease. Eur J Med Chem 169:200–223. https://doi.org/10.1016/j.ejmech.2019.02.076
Alcaro S, Bolognesi ML, García-Sosa AT, Rapposelli S (2019) Editorial: Multi-target-directed ligands (MTDL) as challenging research tools in drug discovery: from design to pharmacological evaluation. Front Chem 7:71. https://doi.org/10.3389/fchem.2019.00071
Muayqil T, Camicioli R (2012) Systematic review and meta-analysis of combination therapy with cholinesterase inhibitors and memantine in Alzheimer’s disease and other dementias. Dement Geriatr Cogn Dis Extra 2:546–572. https://doi.org/10.1159/000343479
Matsunaga S, Kishi T, Iwata N (2015) Combination therapy with cholinesterase inhibitors and memantine for Alzheimer’s disease: a systematic review and meta-analysis. Int J Neuropsychopharmacol 18:1–11. https://doi.org/10.1093/ijnp/pyu115
Namzaric (memantine hydrochloride extended-release/donepezil hydrochloride) Capsules
Al Mamun A, Uddin MS, Kabir MT et al (2020) Exploring the promise of targeting ubiquitin–proteasome system to combat Alzheimer’s disease. Neurotox Res 38:8–17. https://doi.org/10.1007/s12640-020-00185-1
Uddin MS, Devesh T, Al Mamun A et al (2020) Circadian and sleep dysfunction in Alzheimer’s disease. Ageing Res Rev 60:101046. https://doi.org/10.1016/J.ARR.2020.101046
Bloom BS, de Pouvourville N, Straus WL (2003) Cost of illness of Alzheimer’s disease: how useful are current estimates? Gerontologist 43:158–164. https://doi.org/10.1093/geront/43.2.158
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. https://doi.org/10.1101/cshperspect.a006189
Ibrahim AM, Pottoo FH, Dahiya ES et al (2020) Neuron-glia interaction: molecular basis of Alzheimer’s disease and applications of neuroproteomics. Eur J Neurosci. https://doi.org/10.1111/ejn.14838
Habtemariam S (2019) Natural products in Alzheimer’s disease therapy: would old therapeutic approaches fix the broken promise of modern medicines? Molecules 24:1519. https://doi.org/10.3390/molecules24081519
Al Mamun A, Sahab Uddin M, Fahim Bin Bashar M et al (2020) Molecular insight into the therapeutic promise of targeting APOE4 for Alzheimer’s disease. Oxidative Med Cell Longev 2020:5086250–5086216. https://doi.org/10.1155/2020/5086250
Uddin MS, Kabir MT, Rahman MS et al (2020) Revisiting the amyloid cascade hypothesis: from anti-Aβ therapeutics to auspicious new ways for Alzheimer’s disease. Int J Mol Sci 21:5858. https://doi.org/10.3390/ijms21165858
Uddin MS, Kabir MT, Tewari D, Mathew B, Aleya L (2019) Emerging signal regulating potential of small molecule biflavonoids to combat neuropathological insults of Alzheimer’s disease. Sci Total Environ 134836:134836. https://doi.org/10.1016/j.scitotenv.2019.134836
Tanjir Islam M, Sahab Uddin M, Nahar Lucky K et al (2017) Autophagic dysfunction in type 2 diabetes mellitus: pathophysiology and therapeutic implications. J Diabeteas Metab 8:1–8. https://doi.org/10.4172/2155-6156.1000742
Santos RX, Correia SC, Wang X et al (2010) Alzheimer’s disease: diverse aspects of mitochondrial malfunctioning. Int J Clin Exp Pathol 3:570–581
Bhaskar K, Maphis N, Xu G et al (2014) Microglial derived tumor necrosis factor-α drives Alzheimer’s disease-related neuronal cell cycle events. Neurobiol Dis 62:273–285. https://doi.org/10.1016/j.nbd.2013.10.007
Uddin MS, Kabir MT, Al Mamun A et al (2020) Pharmacological approaches to mitigate neuroinflammation in Alzheimer’s disease. Int Immunopharmacol 84:106479. https://doi.org/10.1016/j.intimp.2020.106479
Praticò D (2008) Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci 1147:70–78. https://doi.org/10.1196/annals.1427.010
Uddin MS, Kabir MT (2019) Oxidative stress in Alzheimer’s disease: molecular hallmarks of underlying vulnerability. In: Biological, diagnostic and therapeutic advances in Alzheimer’s disease. Springer Singapore, Singapore, pp. 91–115
Moreira PI, Nunomura A, Nakamura M et al (2008) Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med 44:1493–1505. https://doi.org/10.1016/j.freeradbiomed.2008.01.002
Uddin MS, Al Mamun A, Kabir MT et al (2020) Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur J Pharmacol:173412. https://doi.org/10.1016/j.ejphar.2020.173412
Fukuda M, Kanou F, Shimada N et al (2009) Elevated levels of 4-hydroxynonenal-histidine Michael adduct in the hippocampi of patients with Alzheimer’s disease. Biomed Res 30:227–233. https://doi.org/10.2220/biomedres.30.227
Sultana R, Mecocci P, Mangialasche F, Cecchetti R, Baglioni M, Butterfield DA (2011) Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer’s disease: insights into the role of oxidative stress in Alzheimer’s disease and initial investigations into a potential biomarker for this. J Alzheimers Dis 24:77–84. https://doi.org/10.3233/JAD-2011-101425
Uddin MS, Upaganlawar AB (2019) Oxidative stress and antioxidant defense: biomedical value in health and diseases. Nova Science Publishers, Hauppauge
Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B et al (2012) Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol 69:836–841. https://doi.org/10.1001/archneurol.2012.85
Aliev G, Li Y, Palacios HH, Obrenovich ME (2011) Oxidative stress induced mitochondrial DNA deletion as a hallmark for the drug development in the context of the cerebrovascular diseases. Recent Pat Cardiovasc Drug Discov 6:222–241. https://doi.org/10.2174/157489011797376942
Hayashi T, Shishido N, Nakayama K et al (2007) Lipid peroxidation and 4-hydroxy-2-nonenal formation by copper ion bound to amyloid-β peptide. Free Radic Biol Med 43:1552–1559. https://doi.org/10.1016/j.freeradbiomed.2007.08.013
Nakamura M, Shishido N, Nunomura A et al (2007) Three histidine residues of amyloid-β peptide control the redox activity of copper and iron. Biochemistry 46:12737–12743. https://doi.org/10.1021/bi701079z
Lee YJ, Jeong SY, Karbowski M et al (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, Opa1 in apoptosis. Mol Biol Cell 15:5001–5011. https://doi.org/10.1091/mbc.E04-04-0294
Zhu X, Lee HG, Perry G, Smith MA (2007) Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta Mol basis Dis 1772:494–502. https://doi.org/10.1016/j.bbadis.2006.10.014
Lee HJ, Park MK, Seo YR (2018) Pathogenic mechanisms of heavy metal induced-Alzheimer’s disease. Toxicol Environ Heal Sci 10:1–10. https://doi.org/10.1007/s13530-018-0340-x
Simunkova M, Alwasel SH, Alhazza IM et al (2019) Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch Toxicol 93:2491–2513. https://doi.org/10.1007/s00204-019-02538-y
Guglielmotto M, Giliberto L, Tamagno E, Tabaton M (2010) Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front Aging Neurosci 2. https://doi.org/10.3389/neuro.24.003.2010
Lee HP, Zhu X, Casadesus G et al (2010) Antioxidant approaches for the treatment of Alzheimers disease. Expert Rev Neurother 10:1201–1208. https://doi.org/10.1586/ern.10.74
Khalil M, Teunissen C, Langkammer C (2011) Iron and neurodegeneration in multiple sclerosis. Mult Scler Int 2011:1–6. https://doi.org/10.1155/2011/606807
Uddin MS, Kabir MT, Jeandet P, Mathew B, Ashraf GM, Perveen A, Bin-Jumah MN, Mousa SA et al (2020) Novel anti-Alzheimer’s therapeutic molecules targeting amyloid precursor protein processing. Oxidative Med Cell Longev 2020:1–19. https://doi.org/10.1155/2020/7039138
Liang J, Li J, Jia R et al (2018) Identification of the optimal cognitive drugs among alzheimer’s disease: a Bayesian meta-analytic review. Clin Interv Aging 13:2061–2073. https://doi.org/10.2147/CIA.S184968
Kabir MT, Uddin MS, Al Mamun A et al (2020) Combination drug therapy for the management of Alzheimer’s disease. Int J Mol Sci 21:3272. https://doi.org/10.3390/IJMS21093272
Qizilbash N, Whitehead A, Higgins J, Wilcock G, Schneider L, Farlow M, Dementia Trialists' Collaboration (1998) Cholinesterase inhibition for Alzheimer disease: a meta-analysis of the tacrine trials. J Am Med Assoc 280:1777–1782
Birks JS, Chong LY, Grimley Evans J (2015, 2015) Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev 9:CD001191. https://doi.org/10.1002/14651858.CD001191.pub4
Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL (1999) Structure of acetylcholinesterase complexed with (−)-galanthamine at 2.3 Å resolution. FEBS Lett 463:321–326. https://doi.org/10.1016/S0014-5793(99)01637-3
Dajas-Bailador FA, Heimala K, Wonnacott S (2003) The allosteric potentiation of nicotinic acetylcholine receptors by galantamine is transduced into cellular responses in neurons: Ca2+ signals and neurotransmitter release. Mol Pharmacol 64:1217–1226. https://doi.org/10.1124/mol.64.5.1217
Akk G, Steinbach JH (2005) Galantamine activates muscle-type nicotinic acetylcholine receptors without binding to the acetylcholine-binding site. J Neurosci 25:1992–2001. https://doi.org/10.1523/JNEUROSCI.4985-04.2005
Birks J, Harvey RJ (2006) Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev 6:CD001190. https://doi.org/10.1002/14651858.CD001190.pub3
Nochi S, Asakawa N, Sato T (1995) Kinetic study on the inhibition of acetylcholinesterase by 1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-Yl)methylpiperidine hydrochloride (E2020). Biol Pharm Bull 18:1145–1147. https://doi.org/10.1248/bpb.18.1145
Doody RS, Thomas RG, Farlow M et al (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370:311–321. https://doi.org/10.1056/NEJMoa1312889
Lo D, Grossberg GT (2011) Use of memantine for the treatment of dementia. Expert Rev Neurother 11:1359–1370. https://doi.org/10.1586/ern.11.132
Figueiredo CP, Clarke JR, Ledo JH et al (2013) Memantine rescues transient cognitive impairment caused by high-molecular-weight Aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci 33:9626–9634. https://doi.org/10.1523/JNEUROSCI.0482-13.2013
Willard LB, Hauss-Wegrzyniak B, Danysz W, Wenk GL (2000) The cytotoxicity of chronic neuroinflammation upon basal forebrain cholinergic neurons of rats can be attenuated by glutamatergic antagonism or cyclooxygenase-2 inhibition. Exp Brain Res 134:58–65. https://doi.org/10.1007/s002210000446
Song MS, Rauw G, Baker GB, Kar S (2008) Memantine protects rat cortical cultured neurons against β-amyloid-induced toxicity by attenuating tau phosphorylation. Eur J Neurosci 28:1989–2002. https://doi.org/10.1111/j.1460-9568.2008.06498.x
Hu M, Schurdak ME, Puttfarcken PS, el Kouhen R, Gopalakrishnan M, Li J (2007) High content screen microscopy analysis of Aβ1-42-induced neurite outgrowth reduction in rat primary cortical neurons: neuroprotective effects of α7 neuronal nicotinic acetylcholine receptor ligands. Brain Res 1151:227–235. https://doi.org/10.1016/j.brainres.2007.03.051
Esposito Z, Belli L, Toniolo S et al (2013) Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: are we on the right track? CNS Neurosci Ther 19:549–555. https://doi.org/10.1111/cns.12095
Parsons CG, Danysz W, Dekundy A, Pulte I (2013) Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res 24:358–369
Greenamyre JT, Young AB (1989) Excitatory amino acids and Alzheimer’s disease. Neurobiol Aging 10:593–602
Wenk GL, Danysz W, Mobley SL (1994) Investigations of neurotoxicity and neuroprotection within the nucleus basalis of the rat. Brain Res 655:7–11. https://doi.org/10.1016/0006-8993(94)91590-3
Allen TGJ, Abogadie FC, Brown DA (2006) Simultaneous release of glutamate and acetylcholine from single magnocellular “cholinergic” basal forebrain neurons. J Neurosci 26:1588–1595. https://doi.org/10.1523/JNEUROSCI.3979-05.2006
Nyakas C, Granic I, Halmy LG et al (2011) The basal forebrain cholinergic system in aging and dementia. Rescuing cholinergic neurons from neurotoxic amyloid-β42 with memantine. Behav Brain Res 221:594–603. https://doi.org/10.1016/j.bbr.2010.05.033
Rosini M, Simoni E, Bartolini M et al (2008) Inhibition of acetylcholinesterase, β-amyloid aggregation, and NMDA receptors in Alzheimer’s disease: a promising direction for the multi-target-directed ligands gold rush. J Med Chem 51:4381–4384. https://doi.org/10.1021/jm800577j
Rosini M, Simoni E, Minarini A, Melchiorre C (2014) Multi-target design strategies in the context of Alzheimer’s disease: acetylcholinesterase inhibition and NMDA receptor antagonism as the driving forces. Neurochem Res 39:1914–1923
Geerts H (2006) Pharmacology of acetylcholinesterase inhibitors and N-methyl-D-aspartate receptors for combination therapy in the treatment of Alzheimer’s disease. J Clin Pharmacol 46:8S–16S. https://doi.org/10.1177/0091270006288734
Busquet P, Capurro V, Cavalli A et al (2012) Synergistic effects of galantamine and memantine in attenuating scopolamine-induced amnesia in mice. J Pharmacol Sci 120:305–309. https://doi.org/10.1254/jphs.12166SC
Simoni E, Daniele S, Bottegoni G et al (2012) Combining galantamine and memantine in multitargeted, new chemical entities potentially useful in Alzheimer’s disease. J Med Chem 55:9708–9721. https://doi.org/10.1021/jm3009458
Miller G (2010) The puzzling rise and fall of a dark-horse Alzheimer’s drug. Science 327:1309
Rosini M, Simoni E, Bartolini M et al (2013) The bivalent ligand approach as a tool for improving the in vitro anti-Alzheimer multitarget profile of dimebon. ChemMedChem 8:1276–1281. https://doi.org/10.1002/cmdc.201300263
Makhaeva GF, Lushchekina SV, Boltneva NP et al (2015) Conjugates of γ 3-carbolines and phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer disease. Sci Rep 5:1–11. https://doi.org/10.1038/srep13164
Makhaeva GF, Grigoriev VV, Proshin AN et al (2017) Novel conjugates of tacrine with 1,2,4,-thiadiazole as highly effective cholinesterase inhibitors, blockers of NMDA receptors, and antioxidants. Dokl Biochem Biophys 477:405–409. https://doi.org/10.1134/S1607672917060163
Pérez-Areales FJ, Turcu AL, Barniol-Xicota M et al (2019) A novel class of multitarget anti-Alzheimer benzohomoadamantane–chlorotacrine hybrids modulating cholinesterases and glutamate NMDA receptors. Eur J Med Chem 180:613–626. https://doi.org/10.1016/j.ejmech.2019.07.051
Bachurin SO, Shevtsova EF, Makhaeva GF et al (2017) Novel conjugates of aminoadamantanes with carbazole derivatives as potential multitarget agents for AD treatment. Sci Rep 7:1–15. https://doi.org/10.1038/srep45627
de Candia M, Zaetta G, Denora N, Tricarico D, Majellaro M, Cellamare S, Altomare CD (2017) New azepino[4,3-b]indole derivatives as nanomolar selective inhibitors of human butyrylcholinesterase showing protective effects against NMDA-induced neurotoxicity. Eur J Med Chem 125:288–298. https://doi.org/10.1016/j.ejmech.2016.09.037
Shao D, Zou C, Luo C, Tang X, Li Y (2004) Synthesis and evaluation of tacrine–E2020 hybrids as acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett 14:4639–4642. https://doi.org/10.1016/J.BMCL.2004.07.005
Alonso D, Dorronsoro I, Rubio L et al (2005) Donepezil–tacrine hybrid related derivatives as new dual binding site inhibitors of AChE. Bioorg Med Chem 13:6588–6597. https://doi.org/10.1016/J.BMC.2005.09.029
Camps P, Formosa X, Galdeano C et al (2010) Tacrine-based dual binding site acetylcholinesterase inhibitors as potential disease-modifying anti-Alzheimer drug candidates. Chem Biol Interact 187:411–415. https://doi.org/10.1016/J.CBI.2010.02.013
Camps P, Formosa X, Galdeano C et al (2008) Novel donepezil-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced β-amyloid aggregation. J Med Chem 51:3588–3598. https://doi.org/10.1021/jm8001313
Galdeano C, Viayna E, Sola I et al (2012) Huprine–tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer’s and prion diseases. J Med Chem 55:661–669. https://doi.org/10.1021/jm200840c
Cen J, Guo H, Hong C et al (2018) Development of tacrine–bifendate conjugates with improved cholinesterase inhibitory and pro-cognitive efficacy and reduced hepatotoxicity. Eur J Med Chem 144:128–136. https://doi.org/10.1016/j.ejmech.2017.12.005
Rosini M, Andrisano V, Bartolini M et al (2005) Rational approach to discover multipotent anti-Alzheimer drugs. J Med Chem 48:360–363. https://doi.org/10.1021/jm049112h
González JF, Alcántara AR, Doadrio AL, Sánchez-Montero JM (2019) Developments with multi-target drugs for Alzheimer’s disease: an overview of the current discovery approaches. Expert Opin Drug Discovery 14:879–891
Bolognesi ML, Cavalli A, Bergamini C et al (2009) Toward a rational design of multitarget-directed antioxidants: merging memoquin and lipoic acid molecular frameworks. J Med Chem 52:7883–7886. https://doi.org/10.1021/jm901123n
Rosini M, Simoni E, Bartolini M et al (2011) Exploiting the lipoic acid structure in the search for novel multitarget ligands against Alzheimer’s disease. Eur J Med Chem 46:5435–5442. https://doi.org/10.1016/j.ejmech.2011.09.001
Agatonovic-Kustrin S, Kettle C, Morton DW (2018) A molecular approach in drug development for Alzheimer’s disease. Biomed Pharmacother 106:553–565
Scott JW, Cort WM, Harley H, Parrish DR, Saucy G (1974) 6-Hydroxychroman-2-carboxylic acids: novel antioxidants. J Am Oil Chem Soc 51:200–203. https://doi.org/10.1007/BF02632894
Quintanilla RA, Muñoz FJ, Metcalfe MJ et al (2005) Trolox and 17β-estradiol protect against amyloid β-peptide neurotoxicity by a mechanism that involves modulation of the Wnt signaling pathway. J Biol Chem 280:11615–11625. https://doi.org/10.1074/jbc.M411936200
Thiratmatrakul S, Yenjai C, Waiwut P, Vajragupta O, Reubroycharoen P, Tohda M, Boonyarat C (2014) Synthesis, biological evaluation and molecular modeling study of novel tacrine-carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem 75:21–30. https://doi.org/10.1016/j.ejmech.2014.01.020
Xie SS, Lan JS, Wang XB et al (2015) Multifunctional tacrine-trolox hybrids for the treatment of Alzheimer’s disease with cholinergic, antioxidant, neuroprotective and hepatoprotective properties. Eur J Med Chem 93:42–50. https://doi.org/10.1016/j.ejmech.2015.01.058
Schewe T (1995) Molecular actions of Ebselen-an antiinflammatory antioxidant. Gen Pharmacol 26:1153–1169. https://doi.org/10.1016/0306-3623(95)00003-J
Yamagata K, Ichinose S, Miyashita A, Tagami M (2008) Protective effects of ebselen, a seleno-organic antioxidant on neurodegeneration induced by hypoxia and reperfusion in stroke-prone spontaneously hypertensive rat. Neuroscience 153:428–435. https://doi.org/10.1016/j.neuroscience.2008.02.028
Haratake M, Yoshida S, Mandai M et al (2013) Elevated amyloid-β plaque deposition in dietary selenium-deficient Tg2576 transgenic mice. Metallomics 5:479–483. https://doi.org/10.1039/c3mt00035d
Mao F, Chen J, Zhou Q et al (2013) Novel tacrine-ebselen hybrids with improved cholinesterase inhibitory, hydrogen peroxide and peroxynitrite scavenging activity. Bioorg Med Chem Lett 23:6737–6742. https://doi.org/10.1016/j.bmcl.2013.10.034
Howlett DR, George AR, Owen DE et al (1999) Common structural features determine the effectiveness of carvedilol, daunomycin and rolitetracycline as inhibitors of Alzheimer β-amyloid fibril formation. Biochem J 343:419–423. https://doi.org/10.1042/0264-6021:3430419
Lysko PG, Lysko KA, Webb CL et al (1998) Neuroprotective activities of carvedilol and a hydroxylated derivative. Role of membrane biophysical interactions. Biochem Pharmacol 56:1645–1656. https://doi.org/10.1016/S0006-2952(98)00275-5
Pi R, Mao X, Chao X et al (2012) Tacrine-6-ferulic acid, a novel multifunctional dimer, inhibits amyloid-β-mediated Alzheimer’s disease-associated pathogenesis in vitro and in vivo. PLoS One 7. https://doi.org/10.1371/journal.pone.0031921
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2013) 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Des Devel Ther 7:1157–1178. https://doi.org/10.2147/DDDT.S49763
Farrell RE (2010) RNA methodologies. Elsevier Inc., Amsterdam
Fernández-Bachiller MI, Pérez C, González-Muñoz GC et al (2010) Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimers disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J Med Chem 53:4927–4937. https://doi.org/10.1021/jm100329q
Piazzi L, Rampa A, Bisi A et al (2003) 3-(4-{[benzyl(methyl)amino]methyl}-phenyl)-6,7-dimethoxy-2H-2-chromenone (AP2238) inhibits both acetylcholinesterase and acetylcholinesterase-induced β-amyloid aggregation: a dual function lead for Alzheimer’s disease therapy. J Med Chem 46:2279–2282. https://doi.org/10.1021/jm0340602
Rizzo S, Bartolini M, Ceccarini L, Piazzi L, Gobbi S, Cavalli A, Recanatini M, Andrisano V et al (2010) Targeting Alzheimer’s disease: novel indanone hybrids bearing a pharmacophoric fragment of AP2238. Bioorg Med Chem 18:1749–1760. https://doi.org/10.1016/j.bmc.2010.01.071
Viayna E, Gómez T, Galdeano C et al (2010) Novel huprine derivatives with inhibitory activity toward β-amyloid aggregation and formation as disease-modifying anti-Alzheimer drug candidates. ChemMedChem 5:1855–1870. https://doi.org/10.1002/cmdc.201000322
Bolea I, Juárez-Jiménez J, De Los RC et al (2011) Synthesis, biological evaluation, and molecular modeling of donepezil and N-[(5-(Benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine hybrids as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer’s disease. J Med Chem 54:8251–8270. https://doi.org/10.1021/jm200853t
Esteban G, Allan J, Samadi A et al (2014) Kinetic and structural analysis of the irreversible inhibition of human monoamine oxidases by ASS234, a multi-target compound designed for use in Alzheimer’s disease. Biochim Biophys Acta, Proteins Proteomics 1844:1104–1110. https://doi.org/10.1016/j.bbapap.2014.03.006
Bolea I, Gella A, Monjas L et al (2013) Multipotent, permeable drug ASS234 inhibits Aβ aggregation, possesses antioxidant properties and protects from Aβ-induced apoptosis in vitro. Curr Alzheimer Res 10:797–808. https://doi.org/10.2174/15672050113109990151
Stasiak A, Mussur M, Unzeta M et al (2014) Effects of novel monoamine oxidases and cholinesterases targeting compounds on brain neurotransmitters and behavior in rat model of vascular dementia. Curr Pharm Des 20:161–171. https://doi.org/10.2174/13816128113199990026
Del Pino J, Marco-Contelles J, López-Muñoz F et al (2018) Neuroinflammation signaling modulated by ASS234, a multitarget small molecule for Alzheimer’s disease therapy. ACS Chem Neurosci 9:2880–2885. https://doi.org/10.1021/acschemneuro.8b00203
Zheng H, Youdim MBH, Fridkin M (2009) Site-activated multifunctional chelator with acetylcholinesterase and neuroprotective−neurorestorative moieties for Alzheimer’s therapy. J Med Chem 52:4095–4098. https://doi.org/10.1021/jm900504c
Amit T, Avramovich-Tirosh Y, Youdim MBH, Mandel S (2008) Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J 22:1296–1305. https://doi.org/10.1096/fj.07-8627rev
Gal S, Zheng H, Fridkin M, Youdim MBH (2005) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J Neurochem 95:79–88. https://doi.org/10.1111/j.1471-4159.2005.03341.x
Zheng H, Gal S, Weiner LM et al (2005) Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: in vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J Neurochem 95:68–78. https://doi.org/10.1111/j.1471-4159.2005.03340.x
Sheng R, Lin X, Li J et al (2005) Design, synthesis, and evaluation of 2-phenoxy-indan-1-one derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem Lett 15:3834–3837. https://doi.org/10.1016/J.BMCL.2005.05.132
Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, Nagel J, Lane R (2005) Rivastigmine and donepezil treatment in moderate to moderately-severe Alzheimer’s disease over a 2-year period. Curr Med Res Opin 21:1317–1327. https://doi.org/10.1185/030079905X56565
Lecoutey C, Hedou D, Freret T, Giannoni P, Gaven F, Since M, Bouet V, Ballandonne C et al (2014) Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer’s disease treatment. Proc Natl Acad Sci U S A 111:E3825–E3830. https://doi.org/10.1073/pnas.1410315111
Faye C, Hen R, Guiard BP, Denny CA, Gardier AM, Mendez-David I, David DJ (2020) Rapid anxiolytic effects of RS67333, a serotonin type 4 receptor agonist, and diazepam, a benzodiazepine, are mediated by projections from the prefrontal cortex to the dorsal raphe nucleus. Biol Psychiatry 87:514–525. https://doi.org/10.1016/j.biopsych.2019.08.009
Giannoni P, Gaven F, De Bundel D et al (2013) Early administration of RS 67333, a specific 5-HT4 receptor agonist, prevents amyloidogenesis and behavioral deficits in the 5XFAD mouse model of Alzheimer’s disease. Front Aging Neurosci 5:96. https://doi.org/10.3389/fnagi.2013.00096
Rochais C, Lecoutey C, Gaven F et al (2015) Novel multitarget-directed ligands (MTDLs) with acetylcholinesterase (AChE) inhibitory and serotonergic subtype 4 receptor (5-HT4R) agonist activities as potential agents against Alzheimer’s disease: the design of donecopride. J Med Chem 58:3172–3187. https://doi.org/10.1021/acs.jmedchem.5b00115
Keri RS, Budagumpi S, Pai RK, Balakrishna RG (2014) Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem 78:340–374. https://doi.org/10.1016/j.ejmech.2014.03.047
Hossain MF, Uddin MS, Uddin GMS et al (2019) Melatonin in Alzheimer’s disease: a latent endogenous regulator of neurogenesis to mitigate Alzheimer’s neuropathology. Mol Neurobiol 56:8255–8276
Edmondson DE, Mattevi A, Binda C et al (2012) Structure and mechanism of monoamine oxidase. Curr Med Chem 11:1983–1993. https://doi.org/10.2174/0929867043364784
Tipton KF, Boyce S, O’Sullivan J et al (2012) Monoamine oxidases: certainties and uncertainties. Curr Med Chem 11:1965–1982. https://doi.org/10.2174/0929867043364810
Pachón-Angona I, Refouvelet B, Andrýs R et al (2019) Donepezil + chromone + melatonin hybrids as promising agents for Alzheimer’s disease therapy. J Enzyme Inhib Med Chem 34:479–489. https://doi.org/10.1080/14756366.2018.1545766
Weinreb O, Amit T, Bar-Am O, Youdim MBH (2010) Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog Neurobiol 92:330–344
Weinreb O, Amit T, Riederer P et al (2011) Neuroprotective profile of the multitarget drug rasagiline in Parkinson’s disease. Int Rev Neurobiol 100:127–149. https://doi.org/10.1016/B978-0-12-386467-3.00007-8
Zheng H, Amit T, Bar-Am O et al (2012) From anti-Parkinson’s drug rasagiline to novel multitarget iron chelators with acetylcholinesterase and monoamine oxidase inhibitory and neuroprotective properties for Alzheimer’s disease. J Alzheimers Dis 30:1–16
Weinreb O, Amit T, Bar-Am O, Youdim MBH (2016) Neuroprotective effects of multifaceted hybrid agents targeting MAO, cholinesterase, iron and β-amyloid in ageing and Alzheimer’s disease. Br J Pharmacol 173:2080–2094. https://doi.org/10.1111/bph.13318
Uddin MS, Kabir MT, Rahman MM et al (2020) TV 3326 for Alzheimer’s dementia: a novel multimodal ChE and MAO inhibitors to mitigate Alzheimer’s-like neuropathology. J Pharm Pharmacol 72:1001–1012. https://doi.org/10.1111/jphp.13244
Bar-Am O, Weinreb O, Amit T, Youdim MBH (2009) The novel cholinesterase-monoamine oxidase inhibitor and antioxidant, ladostigil, confers neuroprotection in neuroblastoma cells and aged rats. J Mol Neurosci 37:135–145. https://doi.org/10.1007/s12031-008-9139-6
Weinreb O, Bar-Am O, Amit T et al (2008) The neuroprotective effect of ladostigil against hydrogen peroxide-mediated cytotoxicity. Chem Biol Interact 175:318–326. https://doi.org/10.1016/j.cbi.2008.05.038
Uddin MS, Kabir MT, Rahman MH et al (2020) Exploring the multifunctional neuroprotective promise of rasagiline derivatives for multi-dysfunctional Alzheimer’s disease. Curr Pharm Des 26. https://doi.org/10.2174/1381612826666200406075044
Weinreb O, Amit T, Bar-Am O, Youdim MBH (2007) Induction of neurotrophic factors GDNF and BDNF associated with the mechanism of neurorescue action of rasagiline and ladostigil: new insights and implications for therapy. In: Annals of the New York Academy of Sciences. Blackwell Publishing Inc., pp. 155–168
Schneider LS, Geffen Y, Rabinowitz J et al (2019) Low-dose ladostigil for mild cognitive impairment: a phase 2 placebo-controlled clinical trial. Neurology 93:e1474–e1484. https://doi.org/10.1212/WNL.0000000000008239
Bolognesi ML, Cavalli A, Melchiorre C (2009) Memoquin: a multi-target-directed ligand as an innovative therapeutic opportunity for Alzheimer’s disease. Neurotherapeutics 6:152–162. https://doi.org/10.1016/j.nurt.2008.10.042
Capurro V, Busquet P, Lopes JP et al (2013) Pharmacological characterization of memoquin, a multi-target compound for the treatment of Alzheimer’s disease. PLoS One 8. https://doi.org/10.1371/journal.pone.0056870
Zheng H, Fridkin M, Youdim M (2014) From single target to multitarget/network therapeutics in Alzheimer’s therapy. Pharmaceuticals 7:113–135. https://doi.org/10.3390/ph7020113
Reggiani AM, Simoni E, Caporaso R et al (2016) In vivo characterization of ARN14140, a memantine/galantamine-based multi-target compound for Alzheimer’s disease. Sci Rep 6:1–11. https://doi.org/10.1038/srep33172
Peters O, Fuentes M, Joachim LK et al (2015) Combined treatment with memantine and galantamine-CR compared with galantamine-CR only in antidementia drug naïve patients with mild-to-moderate Alzheimer’s disease. Alzheimers Dement Transl Res Clin Interv 1:198–204. https://doi.org/10.1016/j.trci.2015.10.001
Peters O, Lorenz D, Fesche A et al (2012) A combination of galantamine and memantine modifies cognitive function in subjects with amnestic MCI. J Nutr Health Aging 16:544–548. https://doi.org/10.1007/s12603-012-0062-8
Matsuzono K, Hishikawa N, Ohta Y et al (2015) Combination therapy of cholinesterase inhibitor (donepezil or galantamine) plus memantine in the Okayama memantine study. J Alzheimers Dis 45:771–780. https://doi.org/10.3233/JAD-143084
Singhal M, Merino V, Rosini M et al (2019) Controlled iontophoretic delivery in vitro and in vivo of ARN14140 – a multitarget compound for Alzheimer’s disease. Mol Pharm 16:3460–3468. https://doi.org/10.1021/acs.molpharmaceut.9b00252
Choi YB, Tenneti L, Le DA et al (2000) Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci 3:15–21. https://doi.org/10.1038/71090
Takahashi H, Xia P, Cui J et al (2015) Pharmacologically targeted NMDA receptor antagonism by NitroMemantine for cerebrovascular disease. Sci Rep 5:14781. https://doi.org/10.1038/srep14781
Talantova M, Sanz-Blasco S, Zhang X et al (2013) Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A 110. https://doi.org/10.1073/pnas.1306832110
Kaniakova M, Nepovimova E, Kleteckova L et al (2019) Combination of memantine and 6-chlorotacrine as novel multi-target compound against Alzheimer’s disease. Curr Alzheimer Res 16:821–833. https://doi.org/10.2174/1567205016666190228122218
Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10:698–712. https://doi.org/10.1038/nrd3505
Nguyen L, Lucke-Wold BP, Mookerjee SA et al (2015) Role of sigma-1 receptors in neurodegenerative diseases. J Pharmacol Sci 127:17–29. https://doi.org/10.1016/j.jphs.2014.12.005
Lahmy V, Meunier J, Malmström S et al (2013) Blockade of tau hyperphosphorylation and aβ 1-42 generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and σ 1 receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology 38:1706–1723. https://doi.org/10.1038/npp.2013.70
Besnard J, Ruda GF, Setola V et al (2012) Automated design of ligands to polypharmacological profiles. Nature 492:215–220. https://doi.org/10.1038/nature11691
Al-Ali H, Lee DH, Danzi MC et al (2015) Rational polypharmacology: systematically identifying and engaging multiple drug targets to promote axon growth. ACS Chem Biol 10:1939–1951. https://doi.org/10.1021/acschembio.5b00289
Kumar R, Harilal S, Gupta SV et al (2019) Exploring the new horizons of drug repurposing: a vital tool for turning hard work into smart work. Eur J Med Chem 182:111602. https://doi.org/10.1016/j.ejmech.2019.111602
Kabir MT, Uddin MS, Begum MM et al (2019) Cholinesterase inhibitors for Alzheimer’s disease: multitargeting strategy based on anti-Alzheimer’s drugs repositioning. Curr Pharm Des 25:3519–3535. https://doi.org/10.2174/1381612825666191008103141
Kabir MT, Sufian MA, Uddin MS et al (2019) NMDA receptor antagonists: repositioning of memantine as a multitargeting agent for Alzheimer’s therapy. Curr Pharm Des 25:3506–3518. https://doi.org/10.2174/1381612825666191011102444
Xie SS, Wang XB, Li JY et al (2013) Design, synthesis and evaluation of novel tacrine-coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur J Med Chem 64:540–553. https://doi.org/10.1016/j.ejmech.2013.03.051
Ali MA, Yar MS, Hasan MZ et al (2009) Design, synthesis and evaluation of novel 5,6-dimethoxy-1-oxo-2,3-dihydro-1H-2-indenyl-3,4-substituted phenyl methanone analogues. Bioorg Med Chem Lett 19:5075–5077. https://doi.org/10.1016/j.bmcl.2009.07.042
Khoobi M, Alipour M, Moradi A et al (2013) Design, synthesis, docking study and biological evaluation of some novel tetrahydrochromeno [3′,4′:5,6]pyrano[2,3-b]quinolin-6(7H)-one derivatives against acetyl- and butyrylcholinesterase. Eur J Med Chem 68:291–300. https://doi.org/10.1016/j.ejmech.2013.07.045
Catto M, Pisani L, Leonetti F et al (2013) Design, synthesis and biological evaluation of coumarin alkylamines as potent and selective dual binding site inhibitors of acetylcholinesterase. Bioorg Med Chem 21:146–152. https://doi.org/10.1016/j.bmc.2012.10.045
Jin P, Kim JA, Choi DY, Lee YJ, Jung HS, Hong JT (2013) Anti-inflammatory and anti-amyloidogenic effects of a small molecule, 2,4-bis(p-hydroxyphenyl)-2-butenal in Tg2576 Alzheimer’s disease mice model. J Neuroinflammation 10. https://doi.org/10.1186/1742-2094-10-2
Harilal S, Jose J et al (2020) Revisiting the blood–brain barrier: a hard nut to crack in the transportation of drug molecules. Brain Res Bull. https://doi.org/10.1016/j.brainresbull.2020.03.018
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-Track Research Funding Program. This work was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Author information
Authors and Affiliations
Contributions
M.S.U. conceived the original idea and designed the outlines of the study. M.S.U., A.A.M., and M.T.K. wrote the draft of the manuscript. M.S.U. prepared the figures for the manuscript. G.M.A., M.N.B.-J., and M.M.A.-D. performed the literature review and aided in revising the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uddin, M.S., Al Mamun, A., Kabir, M.T. et al. Multi-Target Drug Candidates for Multifactorial Alzheimer’s Disease: AChE and NMDAR as Molecular Targets. Mol Neurobiol 58, 281–303 (2021). https://doi.org/10.1007/s12035-020-02116-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02116-9