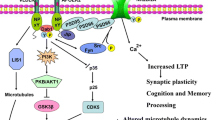
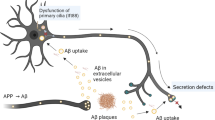
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease that is characterized by progressive memory decline and cognitive dysfunctions. Although the causes of AD have not yet been established, many mechanisms have been proposed. Axon-guidance molecules play the roles in the occurrence and development of AD by participating in different mechanisms. Therefore, what roles do axon-guidance molecules play in AD? This study aimed at elucidating how axon-guidance molecules Netrins, Slits, Semaphorins, and Ephrins regulate the levels of Aβ, hyperphosphorylation of tau protein, Reelin, and other ways through different signaling pathways, in order to show the roles of axon-guidance molecules in the occurrence and development of AD. And it is hoped that this study can provide a theoretical basis and new perspectives in the search for new therapeutic targets for AD.







Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Liu X, Hou D, Lin F, Luo J, Xie J, Wang Y, Tian Y (2019) The role of neurovascular unit damage in the occurrence and development of Alzheimer’s disease. Rev Neurosci 30(5):477–484. https://doi.org/10.1515/revneuro-2018-0056
Zhuang QS, Zheng H, Gu XD, Shen L, Ji HF (2017) Detecting the genetic link between Alzheimer’s disease and obesity using bioinformatics analysis of GWAS data. Oncotarget 8(34)):55915–55919. https://doi.org/10.18632/oncotarget.19115
Liu X, Chen K, Wu T, Weidman D, Lure F, Li J (2018) Use of multimodality imaging and artificial intelligence for diagnosis and prognosis of early stages of Alzheimer’s disease. Transl Res 194:56–67. https://doi.org/10.1016/j.trsl.2018.01.001
Liu Y, Li Z, Ge Q, Lin N, Xiong M (2019) Deep feature selection and causal analysis of Alzheimer’s disease. Front Neurosci 13:1198. https://doi.org/10.3389/fnins.2019.01198
Wang J, Gu BJ, Masters CL, Wang YJ (2017) A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 13(10):612–623. https://doi.org/10.1038/nrneurol.2017.111
Hodson R (2018) Alzheimer’s disease. Nature. 559(7715):S1. https://doi.org/10.1038/d41586-018-05717-6
Guo J, Cheng J, North BJ, Wei W (2017) Functional analyses of major cancer-related signaling pathways in Alzheimer’s disease etiology. Biochim Biophys Acta Rev Cancer 1868(2):341–358. https://doi.org/10.1016/j.bbcan.2017.07.001
Cheng J, North BJ, Zhang T, Dai X, Tao K, Guo J, Wei W (2018) The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell 17(5):e12801. https://doi.org/10.1111/acel.12801
Qiu C, Kivipelto M, von Strauss E (2009) Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 11(2):111–128. https://doi.org/10.31887/DCNS.2009.11.2/cqiu
Kovacs GG (2017) Concepts and classification of neurodegenerative diseases. Handb Clin Neurol 145:301–307. https://doi.org/10.1016/B978-0-12-802395-2.00021-3
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57(4):1105–1121. https://doi.org/10.3233/JAD-161088
Gouras GK, Olsson TT, Hansson O (2015) β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 12(1):3–11. https://doi.org/10.1007/s13311-014-0313-y
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV (2018) The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21(10):1318–1331. https://doi.org/10.1038/s41593-018-0234-x
Yu X, Ji C, Shao A (2020) Neurovascular unit dysfunction and neurodegenerative disorders. Front Neurosci 14:334. https://doi.org/10.3389/fnins.2020.00334
Kandimalla R, Reddy PH (2017) Therapeutics of neurotransmitters in Alzheimer’s disease. J Alzheimers Dis 57(4):1049–1069. https://doi.org/10.3233/JAD-161118
Chong FP, Ng KY, Koh RY, Chye SM (2018) Tau proteins and tauopathies in Alzheimer’s disease. Cell Mol Neurobiol 38(5):965–980. https://doi.org/10.1007/s10571-017-0574-1
Pallo SP, DiMaio J, Cook A, Nilsson B, Johnson GVW (2016) Mechanisms of tau and Aβ-induced excitotoxicity. Brain Res 1634:119–131. https://doi.org/10.1016/j.brainres.2015.12.048
Hurtado MO, Kohler I, de Lange EC (2018) Next-generation biomarker discovery in Alzheimer’s disease using metabolomics - from animal to human studies. Bioanalysis 10(18):1525–1546. https://doi.org/10.4155/bio-2018-0135
Broom GM, Shaw IC, Rucklidge JJ (2019) The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 60:118–121. https://doi.org/10.1016/j.nut.2018.10.003
Kuboyama T, Lee YA, Nishiko H, Tohda C (2015) Inhibition of clathrin-mediated endocytosis prevents amyloid β-induced axonal damage. Neurobiol Aging 36(5):1808–1819. https://doi.org/10.1016/j.neurobiolaging.2015.02.005
Kuboyama T (2018) Visualizing axonal growth cone collapse and early amyloid β effects in cultured mouse neurons. J Vis Exp (140), https://doi.org/10.3791/58229
Rajmohan R, Reddy PH (2017) Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease Neurons. J Alzheimers Dis 57(4):975–999. https://doi.org/10.3233/JAD-160612
Wang Y, Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17(1):5–21. https://doi.org/10.1038/nrn.2015.1
Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121(2):171–181. https://doi.org/10.1007/s00401-010-0789-4
Zhao D, Zhou Y, Huo Y, Meng J, Xiao X, Han L, Zhang X, Luo H et al (2020) RPS23RG1 modulates tau phosphorylation and axon outgrowth through regulating p35 proteasomal degradation. Cell Death Differ. https://doi.org/10.1038/s41418-020-00620-y Advance online publication
Pîrşcoveanu DFV, Pirici I, Tudorică V, Bălşeanu TA, Albu VC, Bondari S, Bumbea AM, Pîrşcoveanu M (2017) Tau protein in neurodegenerative diseases - a review. Romanian J Morphol Embryol 58(4):1141–1150
Jin N, Yin X, Yu D, Cao M, Gong CX, Iqbal K, Ding F, Gu X et al (2015) Truncation and activation of GSK-3β by calpain I: a molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci Rep 5:8187. https://doi.org/10.1038/srep08187
Govindpani K, McNamara LG, Smith NR, Vinnakota C, Waldvogel HJ, Faull RL, Kwakowsky A (2019) Vascular dysfunction in Alzheimer’s Disease: a prelude to the pathological process or a consequence of it? J Clin Med 8(5):651. https://doi.org/10.3390/jcm8050651
Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q (2018) Role of blood-brain barrier in Alzheimer’s disease. J Alzheimers Dis 63(4):1223–1234. https://doi.org/10.3233/JAD-180098
Yamazaki Y, Kanekiyo T (2017) Blood-brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int J Mol Sci 18(9):1965. https://doi.org/10.3390/ijms18091965
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet 388(10043):505–517. https://doi.org/10.1016/S0140-6736(15)01124-1
Romero A, Marco-Contelles J, Ramos E (2020) Highlights of ASS234: a novel and promising therapeutic agent for Alzheimer’s disease therapy. Neural Regen Res 15(1):30–35. https://doi.org/10.4103/1673-5374.262679
McGrattan AM, McGuinness B, McKinley MC, Kee F, Passmore P, Woodside JV, McEvoy CT (2019) Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr Nutr Rep 8(2):53–65. https://doi.org/10.1007/s13668-019-0271-4
Canter RG, Penney J, Tsai LH (2016) The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539(7628):187–196. https://doi.org/10.1038/nature20412
Russell SA, Bashaw GJ (2018) Axon guidance pathways and the control of gene expression. Dev Dyn 247(4):571–580. https://doi.org/10.1002/dvdy.24609
Charron F (2018) Axon guidance: gained in translation. Neuron 99(1):1–2. https://doi.org/10.1016/j.neuron.2018.06.040
Ye X, Qiu Y, Gao Y, Wan D, Zhu H (2019) A subtle network mediating axon guidance: intrinsic dynamic structure of growth cone, attractive and repulsive molecular cues, and the intermediate role of signaling pathways. Neural Plast 2019:1719829. https://doi.org/10.1155/2019/1719829
Stoeckli E (2017) Where does axon guidance lead us? F1000Res 6:78. https://doi.org/10.12688/f1000research.10126.1
Van Battum EY, Brignani S, Pasterkamp RJ (2015) Axon guidance proteins in neurological disorders. Lancet Neurol 14(5):532–546. https://doi.org/10.1016/S1474-4422(14)70257-1
Kim SW, Kim KT (2020) Expression of genes involved in axon guidance: how much have we learned? Int J Mol Sci 21(10):3566. https://doi.org/10.3390/ijms21103566
Yang J, Dong Z, Cui H (2018) Roles and mechanisms of netrins in cancer development. Sheng Wu Gong Cheng Xue Bao 34(6):876–887. https://doi.org/10.13345/j.cjb.170495
Larrieu-Lahargue F, Thomas KR, Li DY (2012) Netrin ligands and receptors: lessons from neurons to the endothelium. Trends Cardiovasc Med 22(2):44–47. https://doi.org/10.1016/j.tcm.2012.06.010
Mukai M, Suruga N, Saeki N, Ogawa K (2017) EphA receptors and ephrin-A ligands are upregulated by monocytic differentiation/maturation and promote cell adhesion and protrusion formation in HL60 monocytes. BMC Cell Biol 18(1):28. https://doi.org/10.1186/s12860-017-0144-x
Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN (1996) frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87(2):197–204. https://doi.org/10.1016/s0092-8674(00)81338-0
Maruyama K, Takemura N, Martino MM, Kondo T, Akira S (2017) Netrins as prophylactic targets in skeletal diseases: a double-edged sword? Pharmacol Res 122:46–52. https://doi.org/10.1016/j.phrs.2017.05.011
Moore SW, Tessier-Lavigne M, Kennedy TE (2007) Netrins and their receptors. Adv Exp Med Biol 621:17–31. https://doi.org/10.1007/978-0-387-76715-4_2
Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M (1996) Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87(2):175–185. https://doi.org/10.1016/s0092-8674(00)81336-7
Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M (1997) Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386(6627):833–838. https://doi.org/10.1038/386833a0
Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E (2008) DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell 133(7):1241–1254. https://doi.org/10.1016/j.cell.2008.05.030
Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chédotal A, Mehlen P (2000) Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature 407(6805):747–750. https://doi.org/10.1038/35037600
Li YN, Pinzón-Duarte G, Dattilo M, Claudepierre T, Koch M, Brunken WJ (2012) The expression and function of netrin-4 in murine ocular tissues. Exp Eye Res 96(1):24–35. https://doi.org/10.1016/j.exer.2012.01.007
Wijeratne DT, Rodger J, Wood FM, Fear MW (2016) The role of Eph receptors and Ephrins in the skin. Int J Dermatol 55(1):3–10. https://doi.org/10.1111/ijd.12968
Wang J, Zheng X, Peng Q, Zhang X, Qin Z (2020) Eph receptors: the bridge linking host and virus. Cell Mol Life Sci 77(12):2355–2365. https://doi.org/10.1007/s00018-019-03409-6
Taylor H, Campbell J, Nobes CD (2017) Ephs and ephrins. Curr Biol 27(3):R90–R95. https://doi.org/10.1016/j.cub.2017.01.003
Barquilla A, Pasquale EB (2015) Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharmacol Toxicol 55:465–487. https://doi.org/10.1146/annurev-pharmtox-011112-140226
O'Leary DD, Wilkinson DG (1999) Eph receptors and ephrins in neural development. Curr Opin Neurobiol 9(1):65–73. https://doi.org/10.1016/s0959-4388(99)80008-7
Alfaro D, Rodríguez-Sosa MR, Zapata AG (2020) Eph/ephrin Signaling and Biology of Mesenchymal Stromal/Stem Cells. J Clin Med 9(2):310. https://doi.org/10.3390/jcm9020310
Blockus H, Chédotal A (2016) Slit-Robo signaling. Development 143(17):3037–3044. https://doi.org/10.1242/dev.132829
Jaworski A, Tom I, Tong RK, Gildea HK, Koch AW, Gonzalez LC, Tessier-Lavigne M (2015) Operational redundancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science 350(6263):961–965. https://doi.org/10.1126/science.aad2615
Yadav SS, Narayan G (2014) Role of ROBO4 signalling in developmental and pathological angiogenesis. Biomed Res Int 2014:683025. https://doi.org/10.1155/2014/683025
Pak JS, DeLoughery ZJ, Wang J, Acharya N, Park Y, Jaworski A, Özkan E (2020) NELL2-Robo3 complex structure reveals mechanisms of receptor activation for axon guidance. Nat Commun 11(1):1489. https://doi.org/10.1038/s41467-020-15211-1
Oleari R, Lettieri A, Paganoni A, Zanieri L, Cariboni A (2019) Semaphorin signaling in GnRH neurons: from development to disease. Neuroendocrinology 109(3):193–199. https://doi.org/10.1159/000495916
Rozbesky D, Robinson RA, Jain V, Renner M, Malinauskas T, Harlos K, Siebold C, Jones EY (2019) Diversity of oligomerization in Drosophila semaphorins suggests a mechanism of functional fine-tuning. Nat Commun 10(1):3691. https://doi.org/10.1038/s41467-019-11683-y
Alto LT, Terman JR (2017) Semaphorins and their signaling mechanisms. Methods Mol Biol 1493:1–25. https://doi.org/10.1007/978-1-4939-6448-2_1
Worzfeld T, Offermanns S (2014) Semaphorins and plexins as therapeutic targets. Nat Rev Drug Discov 13(8):603–621. https://doi.org/10.1038/nrd4337
Nishide M, Kumanogoh A (2018) The role of semaphorins in immune responses and autoimmune rheumatic diseases. Nat Rev Rheumatol 14(1):19–31. https://doi.org/10.1038/nrrheum.2017.201
Neufeld G, Mumblat Y, Smolkin T, Toledano S, Nir-Zvi I, Ziv K, Kessler O (2016) The role of the semaphorins in cancer. Cell Adhes Migr 10(6):652–674. https://doi.org/10.1080/19336918.2016.1197478
Neufeld G, Mumblat Y, Smolkin T, Toledano S, Nir-Zvi I, Ziv K, Kessler O (2016) The semaphorins and their receptors as modulators of tumor progression. Drug Resist Updat 29:1–12. https://doi.org/10.1016/j.drup.2016.08.001
Napolitano V, Tamagnone L (2019) Neuropilins controlling cancer therapy responsiveness. Int J Mol Sci 20(8):2049. https://doi.org/10.3390/ijms20082049
Horch HW, Spicer SB, Low IIC, Joncas CT, Quenzer ED, Okoya H, Ledwidge LM, Fisher HP (2020) Characterization of plexinA and two distinct semaphorin1a transcripts in the developing and adult cricket Gryllus bimaculatus. J Comp Neurol 528(4):687–702. https://doi.org/10.1002/cne.24790
Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, Lackmann M, Boyd AW (2012) Eph/Ephrin signaling in injury and inflammation. Am J Pathol 181(5):1493–1503. https://doi.org/10.1016/j.ajpath.2012.06.043
Merlos-Suárez A, Batlle E (2008) Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol 20(2):194–200. https://doi.org/10.1016/j.ceb.2008.01.011
Bundesen LQ, Scheel TA, Bregman BS, Kromer LF (2003) Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci 23(21):7789–7800. https://doi.org/10.1523/JNEUROSCI.23-21-07789.2003
Fu AK, Ip NY (2007) Cyclin-dependent kinase 5 links extracellular cues to actin cytoskeleton during dendritic spine development. Cell Adhes Migr 1(2):110–112. https://doi.org/10.4161/cam.1.2.4617
Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB (2003) Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci 6(2):153–160. https://doi.org/10.1038/nn994
Irie F, Yamaguchi Y (2004) EPHB receptor signaling in dendritic spine development. Front Biosci 9:1365–1373. https://doi.org/10.2741/1325
Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV (2012) Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci 15(10):1399–1406. https://doi.org/10.1038/nn.3212
Muckom R, McFarland S, Yang C, Perea B, Gentes M, Murugappan A, Tran E, Dordick JS et al (2019) High-throughput combinatorial screening reveals interactions between signaling molecules that regulate adult neural stem cell fate. Biotechnol Bioeng 116(1):193–205. https://doi.org/10.1002/bit.26815
Goldshmit Y, McLenachan S, Turnley A (2006) Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev 52(2):327–345. https://doi.org/10.1016/j.brainresrev.2006.04.006
Bradford D, Cole SJ, Cooper HM (2009) Netrin-1: diversity in development. Int J Biochem Cell Biol 41(3):487–493. https://doi.org/10.1016/j.biocel.2008.03.014
Barallobre MJ, Pascual M, Del Río JA, Soriano E (2005) The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain research. Brain Res Brain Res Rev 49(1):22–47. https://doi.org/10.1016/j.brainresrev.2004.11.003
Xie Y, Hong Y, Ma XY, Ren XR, Ackerman S, Mei L, Xiong WC (2006) DCC-dependent phospholipase C signaling in netrin-1-induced neurite elongation. J Biol Chem 281(5):2605–2611. https://doi.org/10.1074/jbc.M512767200
Glasgow SD, Ruthazer ES, Kennedy TE (2020) Guiding synaptic plasticity: novel roles for netrin-1 in synaptic plasticity and memory formation in the adult brain. J Physiol. Advance online publication. https://doi.org/10.1113/JP278704
Fan Y, Shen F, Chen Y, Hao Q, Liu W, Su H, Young WL, Yang GY (2008) Overexpression of netrin-1 induces neovascularization in the adult mouse brain. J Cereb Blood Flow Metab 28(9):1543–1551. https://doi.org/10.1038/jcbfm.2008.39
Dun XP, Parkinson DB (2017) Role of Netrin-1 signaling in nerve regeneration. Int J Mol Sci 18(3):491. https://doi.org/10.3390/ijms18030491
Manitt C, Wang D, Kennedy TE (2006) Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J Neurosci Res 84(8):1808–1820. https://doi.org/10.1002/jnr.21070
Borrell V, Cárdenas A, Ciceri G, Galcerán J, Flames N, Pla R, Nóbrega-Pereira S, García-Frigola C et al (2012) Slit/Robo signaling modulates the proliferation of central nervous system progenitors. Neuron 76(2):338–352. https://doi.org/10.1016/j.neuron.2012.08.003
Sasaki T, Komatsu Y, Yamamori T (2020) Expression patterns of SLIT/ROBO mRNAs reveal a characteristic feature in the entorhinal-hippocampal area of macaque monkeys. BMC Res Notes 13(1):262. https://doi.org/10.1186/s13104-020-05100-7
Xu C, Fan CM (2008) Expression of Robo/Slit and Semaphorin/Plexin/Neuropilin family members in the developing hypothalamic paraventricular and supraoptic nuclei. Gene Expr Patterns 8(7-8):502–507. https://doi.org/10.1016/j.gep.2008.06.003
Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D (2009) The many faces of semaphorins: from development to pathology. Cell Mol Life Sci 66(4):649–666. https://doi.org/10.1007/s00018-008-8518-z
Mann F, Chauvet S, Rougon G (2007) Semaphorins in development and adult brain: Implication for neurological diseases. Prog Neurobiol 82(2):57–79. https://doi.org/10.1016/j.pneurobio.2007.02.011
Jongbloets BC, Pasterkamp RJ (2014) Semaphorin signalling during development. Development 141(17):3292–3297. https://doi.org/10.1242/dev.105544
Yu HH, Kolodkin AL (1999) Semaphorin signaling: a little less per-plexin. Neuron 22(1):11–14. https://doi.org/10.1016/s0896-6273(00)80672-8
Kolodkin AL, Tessier-Lavigne M (2011) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3(6):a001727. https://doi.org/10.1101/cshperspect.a001727
McDermott JE, Goldblatt D, Paradis S (2018) Class 4 Semaphorins and Plexin-B receptors regulate GABAergic and glutamatergic synapse development in the mammalian hippocampus. Mol Cell Neurosci 92:50–66. https://doi.org/10.1016/j.mcn.2018.06.008
Mata A, Gil V, Pérez-Clausell J, Dasilva M, González-Calixto MC, Soriano E, García-Verdugo JM, Sanchez-Vives MV et al (2018) New functions of Semaphorin 3E and its receptor PlexinD1 during developing and adult hippocampal formation. Sci Rep 8(1):1381. https://doi.org/10.1038/s41598-018-19794-0
Meléndez-Herrera E, Colín-Castelán D, Varela-Echavarría A, Gutiérrez-Ospina G (2008) Semaphorin-3A and its receptor neuropilin-1 are predominantly expressed in endothelial cells along the rostral migratory stream of young and adult mice. Cell Tissue Res 333(2):175–184. https://doi.org/10.1007/s00441-008-0643-3
Sun T, Li W, Ling S (2016) miR-30c and semaphorin 3A determine adult neurogenesis by regulating proliferation and differentiation of stem cells in the subventricular zones of mouse. Cell Prolif 49(3):270–280. https://doi.org/10.1111/cpr.12261
de Wit J, Verhaagen J (2003) Role of semaphorins in the adult nervous system. Prog Neurobiol 71(2-3):249–267. https://doi.org/10.1016/j.pneurobio.2003.06.001
Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, Ginty DD, Kolodkin AL (2005) Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci 25(14):3613–3620. https://doi.org/10.1523/JNEUROSCI.5255-04.2005
Simonetti M, Paldy E, Njoo C, Bali KK, Worzfeld T, Pitzer C, Kuner T, Offermanns S et al (2019) The impact of Semaphorin 4C/Plexin-B2 signaling on fear memory via remodeling of neuronal and synaptic morphology. Mol Psychiatry. https://doi.org/10.1038/s41380-019-0491-4 Advance online publication
Shelly M, Cancedda L, Lim BK, Popescu AT, Cheng PL, Gao H, Poo MM (2011) Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron 71(3):433–446. https://doi.org/10.1016/j.neuron.2011.06.041
Dityatev A, Bukalo O, Schachner M (2008) Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron Glia Biol 4(3):197–209. https://doi.org/10.1017/S1740925X09990111
Lourenço FC, Galvan V, Fombonne J, Corset V, Llambi F, Müller U, Bredesen DE, Mehlen P (2009) Netrin-1 interacts with amyloid precursor protein and regulates amyloid-beta production. Cell Death Differ 16(5):655–663. https://doi.org/10.1038/cdd.2008.191
Li JC, Han L, Wen YX, Yang YX, Li S, Li XS, Zhao CJ, Wang TY et al (2015) Increased permeability of the blood-brain barrier and Alzheimer’s disease-like alterations in slit-2 transgenic mice. J Alzheimers Dis 43(2):535–548. https://doi.org/10.3233/JAD-141215
Yusufov M, Weyandt LL, Piryatinsky I (2017) Alzheimer’s disease and diet: a systematic review. Int J Neurosci 127(2):161–175. https://doi.org/10.3109/00207454.2016.1155572
Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ (2019) Ketogenic Diet in Alzheimer's Disease. Int J Mol Sci 20(16):3892. https://doi.org/10.3390/ijms20163892
Kalani A, Chaturvedi P, Kalani K, Kamat PK, Chaturvedi P (2019) A high methionine, low folate and vitamin B6/B12 containing diet can be associated with memory loss by epigenetic silencing of netrin-1. Neural Regen Res 14(7):1247–1254. https://doi.org/10.4103/1673-5374.251333
Kamat PK, Kyles P, Kalani A, Tyagi N (2016) Hydrogen sulfide ameliorates homocysteine-induced Alzheimer’s disease-like pathology, blood-brain barrier disruption, and synaptic disorder. Mol Neurobiol 53(4):2451–2467. https://doi.org/10.1007/s12035-015-9212-4
Hashimoto Y, Toyama Y, Kusakari S, Nawa M, Matsuoka M (2016) An Alzheimer disease-linked rare mutation potentiates netrin receptor uncoordinated-5c-induced signaling that merges with amyloid β precursor protein signaling. J Biol Chem 291(23):12282–12293. https://doi.org/10.1074/jbc.M115.698092
Sun JH, Wang HF, Zhu XC, Yu WJ, Tan CC, Jiang T, Tan MS, Tan L et al (2016) The impact of UNC5C genetic variations on neuroimaging in Alzheimer’s disease. Mol Neurobiol 53(10):6759–6767. https://doi.org/10.1007/s12035-015-9589-0
Korvatska O, Leverenz JB, Jayadev S, Mc Millan P, Kurtz I, Guo X, Rumbaugh M, Matsushita M et al (2015) R47H Variant of TREM2 Associated with Alzheimer disease in a large late-onset family: clinical, genetic, and neuropathological study. JAMA Neurol 72(8):920–927. https://doi.org/10.1001/jamaneurol.2015.0979
McLarnon JG, Ryu JK (2008) Relevance of abeta1-42 intrahippocampal injection as an animal model of inflamed Alzheimer’s disease brain. Curr Alzheimer Res 5(5):475–480. https://doi.org/10.2174/156720508785908874
Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M et al (2010) Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330(6006):980–982. https://doi.org/10.1126/science.1194516
Sun L, Ju T, Wang T, Zhang L, Ding F, Zhang Y, An R, Sun Y et al (2019) Decreased Netrin-1 and correlated Th17/Tregs balance disorder in Aβ1-42 induced Alzheimer’s disease model rats. Front Aging Neurosci 11:124. https://doi.org/10.3389/fnagi.2019.00124
Spilman PR, Corset V, Gorostiza O, Poksay KS, Galvan V, Zhang J, Rao R, Peters-Libeu C et al (2016) Netrin-1 interrupts amyloid-β amplification, increases sAβPPα in vitro and in vivo, and improves cognition in a mouse model of Alzheimer’s disease. J Alzheimers Dis 52(1):223–242. https://doi.org/10.3233/JAD-151046
Zamani E, Parviz M, Roghani M, Mohseni-Moghaddam P (2019) Key mechanisms underlying netrin-1 prevention of impaired spatial and object memory in Aβ1-42 CA1-injected rats. Clin Exp Pharmacol Physiol 46(1):86–93. https://doi.org/10.1111/1440-1681.13020
Shabani M, Haghani M, Tazangi PE, Bayat M, Shid Moosavi SM, Ranjbar H (2017) Netrin-1 improves the amyloid-β-mediated suppression of memory and synaptic plasticity. Brain Res Bull 131:107–116. https://doi.org/10.1016/j.brainresbull.2017.03.015
Fu AK, Hung KW, Huang H, Gu S, Shen Y, Cheng EY, Ip FC, Huang X et al (2014) Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A 111(27):9959–9964. https://doi.org/10.1073/pnas.1405803111
Kashyap G, Bapat D, Das D, Gowaikar R, Amritkar RE, Rangarajan G, Ravindranath V, Ambika G (2019) Synapse loss and progress of Alzheimer’s disease -a network model. Sci Rep 9(1):6555. https://doi.org/10.1038/s41598-019-43076-y
Huang TY, Zhao Y, Jiang LL, Li X, Liu Y, Sun Y, Piña-Crespo JC, Zhu B et al (2017) SORLA attenuates EphA4 signaling and amyloid β-induced neurodegeneration. J Exp Med 214(12):3669–3685. https://doi.org/10.1084/jem.20171413
Vargas LM, Cerpa W, Muñoz FJ, Zanlungo S, Alvarez AR (2018) Amyloid-β oligomers synaptotoxicity: the emerging role of EphA4/c-Abl signaling in Alzheimer's disease. Biochim Biophys Acta Mol basis Dis 1864(4 Pt A):1148–1159. https://doi.org/10.1016/j.bbadis.2018.01.023
Poppe L, Rué L, Timmers M, Lenaerts A, Storm A, Callaerts-Vegh Z, Courtand G, de Boer A et al (2019) EphA4 loss improves social memory performance and alters dendritic spine morphology without changes in amyloid pathology in a mouse model of Alzheimer’s disease. Alzheimers Res Ther 11(1):102. https://doi.org/10.1186/s13195-019-0554-4
Gu S, Fu WY, Fu AKY, Tong EPS, Ip FCF, Huang X, Ip NY (2018) Identification of new EphA4 inhibitors by virtual screening of FDA-approved drugs. Sci Rep 8(1):7377. https://doi.org/10.1038/s41598-018-25790-1
Chen Q, Song H, Liu C, Xu J, Wei C, Wang W, Han F (2020) The interaction of EphA4 With PDGFRβ regulates proliferation and neuronal differentiation of neural progenitor cells in vitro and promotes neurogenesis in vivo. Front Aging Neurosci 12:7. https://doi.org/10.3389/fnagi.2020.00007
Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11(5):339–350. https://doi.org/10.1038/nrn2822
Tissir F, Goffinet AM (2003) Reelin and brain development. Nat Rev Neurosci 4(6):496–505. https://doi.org/10.1038/nrn1113
Botella-López A, Burgaya F, Gavín R, García-Ayllón MS, Gómez-Tortosa E, Peña-Casanova J, Ureña JM, Del Río JA et al (2006) Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc Natl Acad Sci U S A 103(14):5573–5578. https://doi.org/10.1073/pnas.0601279103
Sentürk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A (2011) Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature 472(7343):356–360. https://doi.org/10.1038/nature09874
Hernández DE, Salvadores NA, Moya-Alvarado G, Catalán RJ, Bronfman FC, Court FA (2018) Axonal degeneration induced by glutamate excitotoxicity is mediated by necroptosis. J Cell Sci 131(22):jcs214684. https://doi.org/10.1242/jcs.214684
Mayor D, Tymianski M (2018) Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology 134(Pt B):178–188. https://doi.org/10.1016/j.neuropharm.2017.11.050
Giau VV, Bagyinszky E, Youn YC, An SSA, Kim S (2019) APP, PSEN1, and PSEN2 Mutations in Asian patients with early-onset Alzheimer disease. Int J Mol Sci 20(19):4757. https://doi.org/10.3390/ijms20194757
Barthet G, Dunys J, Shao Z, Xuan Z, Ren Y, Xu J, Arbez N, Mauger G et al (2013) Presenilin mediates neuroprotective functions of ephrinB and brain-derived neurotrophic factor and regulates ligand-induced internalization and metabolism of EphB2 and TrkB receptors. Neurobiol Aging 34(2):499–510. https://doi.org/10.1016/j.neurobiolaging.2012.02.024
Jaworski T, Banach-Kasper E, Gralec K (2019) GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast 2019:4209475. https://doi.org/10.1155/2019/4209475
Zhang Y, Huang NQ, Yan F, Jin H, Zhou SY, Shi JS, Jin F (2018) Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav Brain Res 339:57–65. https://doi.org/10.1016/j.bbr.2017.11.015
Hernandez F, Lucas JJ, Avila J (2013) GSK3 and tau: two convergence points in Alzheimer’s disease. J Alzheimers Dis 33(Suppl 1):S141–S144. https://doi.org/10.3233/JAD-2012-129025
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104(6):1433–1439. https://doi.org/10.1111/j.1471-4159.2007.05194.x
Jiang J, Wang ZH, Qu M, Gao D, Liu XP, Zhu LQ, Wang JZ (2015) Stimulation of EphB2 attenuates tau phosphorylation through PI3K/Akt-mediated inactivation of glycogen synthase kinase-3β. Sci Rep 5:11765. https://doi.org/10.1038/srep11765
Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M et al (2010) Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem 285(10):7619–7632. https://doi.org/10.1074/jbc.M109.057182
Tamura H, Kawata M, Hamaguchi S, Ishikawa Y, Shiosaka S (2012) Processing of neuregulin-1 by neuropsin regulates GABAergic neuron to control neural plasticity of the mouse hippocampus. J Neurosci 32(37):12657–12672. https://doi.org/10.1523/JNEUROSCI.2542-12.2012
Tamura H, Ishikawa Y, Hino N, Maeda M, Yoshida S, Kaku S, Shiosaka S (2006) Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo. J Physiol 570(Pt 3):541–551. https://doi.org/10.1113/jphysiol.2005.098715
Teuber-Hanselmann S, Rekowski J, Vogelgsang J, von Arnim C, Reetz K, Stang A, Jöckel KH, Wiltfang J et al (2020) CSF and blood Kallikrein-8: a promising early biomarker for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 91(1):40–48. https://doi.org/10.1136/jnnp-2019-321073
Herring A, Münster Y, Akkaya T, Moghaddam S, Deinsberger K, Meyer J, Zahel J, Sanchez-Mendoza E et al (2016) Kallikrein-8 inhibition attenuates Alzheimer’s disease pathology in mice. Alzheimers Dement 12(12):1273–1287. https://doi.org/10.1016/j.jalz.2016.05.006
Münster Y, Keyvani K, Herring A (2020) Inhibition of excessive kallikrein-8 improves neuroplasticity in Alzheimer’s disease mouse model. Exp Neurol 324:113115. https://doi.org/10.1016/j.expneurol.2019.113115
Sadleir KR, Popovic J, Vassar R (2018) ER stress is not elevated in the 5XFAD mouse model of Alzheimer's disease. J Biol Chem 293(48):18434–18443. https://doi.org/10.1074/jbc.RA118.005769
Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E (2014) Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging 35(8):1792–1800. https://doi.org/10.1016/j.neurobiolaging.2014.02.012
Kim S, Nam Y, Jeong YO, Park HH, Lee SK, Shin SJ, Jung H, Kim BH et al (2019) Topographical visualization of the reciprocal projection between the medial septum and the hippocampus in the 5XFAD mouse model of Alzheimer’s disease. Int J Mol Sci 20(16):3992. https://doi.org/10.3390/ijms20163992
Bai B, Wang X, Li Y, Chen PC, Yu K, Dey KK, Yarbro JM, Han X et al (2020) Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105(6):975–991.e7. https://doi.org/10.1016/j.neuron.2019.12.015
Wang H, Dey KK, Chen PC, Li Y, Niu M, Cho JH, Wang X, Bai B et al (2020) Integrated analysis of ultra-deep proteomes in cortex, cerebrospinal fluid and serum reveals a mitochondrial signature in Alzheimer’s disease. Mol Neurodegener 15(1):43. https://doi.org/10.1186/s13024-020-00384-6
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H et al (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76(6):845–861. https://doi.org/10.1002/ana.24271
Bakker EN, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AW, Weller RO, Carare RO (2016) Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 36(2):181–194. https://doi.org/10.1007/s10571-015-0273-8
Li G, He X, Li H, Wu Y, Guan Y, Liu S, Jia H, Li Y et al (2018) Overexpression of Slit2 improves function of the paravascular pathway in the aging mouse brain. Int J Mol Med 42(4):1935–1944. https://doi.org/10.3892/ijmm.2018.3802
Good PF, Alapat D, Hsu A, Chu C, Perl D, Wen X, Burstein DE, Kohtz DS (2004) A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer’s disease. J Neurochem 91(3):716–736. https://doi.org/10.1111/j.1471-4159.2004.02766.x
Villa C, Venturelli E, Fenoglio C, De Riz M, Scalabrini D, Cortini F, Serpente M, Cantoni C et al (2010) Candidate gene analysis of semaphorins in patients with Alzheimer’s disease. Neurol Sci 31(2):169–173. https://doi.org/10.1007/s10072-009-0200-1
Quach TT, Honnorat J, Kolattukudy PE, Khanna R, Duchemin AM (2015) CRMPs: critical molecules for neurite morphogenesis and neuropsychiatric diseases. Mol Psychiatry 20(9):1037–1045. https://doi.org/10.1038/mp.2015.77
Schmidt EF, Strittmatter SM (2007) The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol 600:1–11. https://doi.org/10.1007/978-0-387-70956-7_1
Isono T, Yamashita N, Obara M, Araki T, Nakamura F, Kamiya Y, Alkam T, Nitta A et al (2013) Amyloid-β25-35 induces impairment of cognitive function and long-term potentiation through phosphorylation of collapsin response mediator protein 2. Neurosci Res 77(3):180–185. https://doi.org/10.1016/j.neures.2013.08.005
Jun G, Asai H, Zeldich E, Drapeau E, Chen C, Chung J, Park JH, Kim S et al (2014) PLXNA4 is associated with Alzheimer disease and modulates tau phosphorylation. Ann Neurol 76(3):379–392. https://doi.org/10.1002/ana.24219
Nakamura H, Takahashi-Jitsuki A, Makihara H, Asano T, Kimura Y, Nakabayashi J, Yamashita N, Kawamoto Y et al (2018) Proteome and behavioral alterations in phosphorylation-deficient mutant Collapsin Response Mediator Protein2 knock-in mice. Neurochem Int 119:207–217. https://doi.org/10.1016/j.neuint.2018.04.009
Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K et al (2005) Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer's disease. Genes Cells 10(2):165–179. https://doi.org/10.1111/j.1365-2443.2005.00827.x
Wei W, Wang Y, Wang Y, Dong J, Min H, Song B, Teng W, Xi Q et al (2013) Developmental hypothyroxinaemia induced by maternal mild iodine deficiency delays hippocampal axonal growth in the rat offspring. J Neuroendocrinol 25(9):852–862. https://doi.org/10.1111/jne.12058
Puthiyedth N, Riveros C, Berretta R, Moscato P (2016) Identification of differentially expressed genes through integrated study of Alzheimer’s disease affected brain regions. PLoS One 11(4):e0152342. https://doi.org/10.1371/journal.pone.0152342
Schott JM, Crutch SJ, Carrasquillo MM, Uphill J, Shakespeare TJ, Ryan NS, Yong KX, Lehmann M et al (2016) Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement 12(8):862–871. https://doi.org/10.1016/j.jalz.2016.01.010
Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC (2012) Posterior cortical atrophy. Lancet Neurol 11(2):170–178. https://doi.org/10.1016/S1474-4422(11)70289-7
Shen JN, Wang DS, Wang R (2012) The protection of acetylcholinesterase inhibitor on β-amyloid-induced the injury of neurite outgrowth via regulating axon guidance related genes expression in neuronal cells. Int J Clin Exp Pathol 5(9):900–913
Yang S, Hilton S, Alves JN, Saksida LM, Bussey T, Matthews RT, Kitagawa H, Spillantini MG et al (2017) Antibody recognizing 4-sulfated chondroitin sulfate proteoglycans restores memory in tauopathy-induced neurodegeneration. Neurobiol Aging 59:197–209. https://doi.org/10.1016/j.neurobiolaging.2017.08.002
Barão S, Gärtner A, Leyva-Díaz E, Demyanenko G, Munck S, Vanhoutvin T, Zhou L, Schachner M et al (2015) Antagonistic effects of BACE1 and APH1B-γ-secretase control axonal guidance by regulating growth cone collapse. Cell Rep 12(9):1367–1376. https://doi.org/10.1016/j.celrep.2015.07.059
Nam KN, Mounier A, Fitz NF, Wolfe C, Schug J, Lefterov I, Koldamova R (2016) RXR controlled regulatory networks identified in mouse brain counteract deleterious effects of Aβ oligomers. Sci Rep 6:24048. https://doi.org/10.1038/srep24048
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25(1):59–70. https://doi.org/10.1111/ene.13439
Zhu JB, Tan CC, Tan L, Yu JT (2017) State of play in Alzheimer’s disease genetics. J Alzheimers Dis 58(3):631–659. https://doi.org/10.3233/JAD-170062
Forloni G, Balducci C (2018) Alzheimer’s disease, oligomers, and inflammation. J Alzheimers Dis JAD 62(3):1261–1276. https://doi.org/10.3233/JAD-170819
Costandi M (2018) Ways to stop the spread of Alzheimer’s disease. Nature 559(7715):S16–S17. https://doi.org/10.1038/d41586-018-05723-8
Davda N, Corkill R (2020) Biomarkers in the diagnosis and prognosis of Alzheimer’s disease. J Neurol 267(8):2475–2477. https://doi.org/10.1007/s00415-020-10037-9
Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T et al (2012) Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 366(10):893–903. https://doi.org/10.1056/NEJMoa1106668
Grossberg GT, Manes F, Allegri RF, Gutiérrez-Robledo LM, Gloger S, Xie L, Jia XD, Pejović V et al (2013) The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs 27(6):469–478. https://doi.org/10.1007/s40263-013-0077-7
Weller J, Budson A (2018) Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res 7:F1000 Faculty Rev-1161. https://doi.org/10.12688/f1000research.14506.1
Acknowledgements
We would like to acknowledge Haiying Wang, Bingchen Liu, and Xuda Liu for their valuable advices.
Funding
This work was supported by the Natural Science Foundation of Liaoning Province [2020-MS-152]; the Basic Research Fund of Young Program of Higher Education of Liaoning Province [LQNK201735]; the National Natural Science Foundation of China [No. 81302406]; and the Funds for Distinguished Young Scientists in School of Public Health, China Medical University.
Author information
Authors and Affiliations
Contributions
Each author substantially contributed to the review. Lei Zhang, conception and design and drafting the manuscript; Zhipeng Qi, Jiashuo Li, Minghui Li, Xianchao Du, Shuang Wang, Bin Xu, Wei Liu, Zhaofa Xu, Guoyu Zhou, and Shuhua Xi, revising the manuscript; Yu Deng, conception and design, revising it critically for important intellectual content, and final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Qi, Z., Li, J. et al. Roles and Mechanisms of Axon-Guidance Molecules in Alzheimer’s Disease. Mol Neurobiol 58, 3290–3307 (2021). https://doi.org/10.1007/s12035-021-02311-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02311-2