Abstract
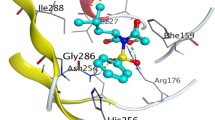
New quinoline derivatives containing biphenyl ring were synthesized and characterized by IR, 1H NMR and mass spectral studies. The synthesized compounds were screened for antimicrobial, anthelmintic activities as well as free radical scavenging property against the DPPH radical. The minimum inhibition concentration values showed promising inhibiting activity and are potent biological agents. The compounds showed minimum binding energy towards β-tubulin. The compounds 11a, 11c, 13c and 13d have good affinity towards the active pocket and may be considered as a good inhibitor of β-tubulin.

New quinoline derivatives containing biphenyl were synthesized from 3-formyl-2-hydroxy-quinoline and screened for antimicrobial, anthelmintic, free radical scavenging activities (DPPH method) and docking against β-tubulin.






Similar content being viewed by others
References
Levy S and Azoulay S 1994 J. Cardiovas. Electrophysiol. 5 635
Wenckebach K F 1923 J. Am. Med. Assoc. 81 472
(a) Bilker O, Lindo V, Panico M, Etiene A E, Paxton T, Dell A, Rogers M, Sinden R E and Morris H R 1998 Nature 392 289; (b) Roma G, Braccio M D, Grossi G, Mattioli F and Ghia H 2000 Eur. J. Med. Chem. 35 1021; (c) Chen Y L, Fang K C, Sheu J Y, Hsu S L and Tzeng C C 2000 J. Med. Chem. 44 2374; (d) Winstanley P A 2000 Parasitol. Today 16 146
(a) Fang K C, Chen Y L, Sheu J Y, Wang T C and Tzeng C C 2000 J. Med. Chem. 43 3809; (b) Chevalier J, Atifi S, Eyraud A, Mahamoud A, Barbe J and Pages J M 2001 J. Med. Chem. 44 4023; (c) Phan L T, Jian T, Chen Z, Qiu Y L, Wang Z, Beach T, Polemeropoulos A and Or Y S 2004 J. Med. Chem. 47 2965; (d) Benkovic S J, Baker S J, Alley M R K, Woo Y H, Zhang Y K, Akama T, Mao W, Baboval J, Rajagopalan P T R, Wall M, Kahng L S, Tavassoli A and Shapiro L 2005 J. Med. Chem. 48 7468
(a) Majerz-Maniecka K, Oleksyn B, Musiol R, Podeszwa B and Polanski J In Abstracts of Papers, Joint Meeting on Medicinal Chemistry, Vienna, Austria 2005, Sci. Pharm. 73 194; (b) Vargas L Y, Castelli M V, Kouznetsov V V, Urbina J M, Lopez S N, Sortino M, Enriz R D, Ribas J C and Zacchino S 2003 Bioorg. Med. Chem. 11 1531; (c) Singh M, Singh M P and Ablordeppey S Y 1996 Drug Dev. Ind. Pharm. 22 377
(a) Dassonneville L, Lansiaux A, Wattelet A, Wattez N, Mahieu C, Van Miert S, Pieters L and Bailly C 2000 Eur. J. Pharmacol. 409 9; (b) Dassonneville L, Bonjean K, De Pauw-Gillet, Colson M C, Houssier P, Quetin-Leclercq C, Angenot J and Ablordeppey L S Y 2002 Bioorg. Med. Chem. 10 1337; (c) Bailly C 1999 Biochemistry 38 7719; (d) Bailly C, Laine W, Baldeyrou B, De Pauw-Gillet M -C, Colson P, Houssier C, Cimanga K, Miert S V, Vlietinck A J and Pieters L 2000 AntiCancer Drug Des. 15 191
(a) Jones G 1996 Comprehensive heterocyclic chemistry II (eds) A R Katritzky, C W Rees and E F Scriven (Pergamon: Oxford) 5, 167; (b) Holla B S, Mahalinga M, Karthikeyan M S, Akberalib P M and Shettyc N S 2006 Bioorg. Med. Chem. 14 2040
(a) Smirnov R F, Tikhomirov B I, Marinchenko G V and Yakubchik A I 1973 Polym. Sci. U.S.S.R. 15 832; (b) Całus S, Gondek E, Danel A, Jarosz B, Pokładko M and Kityk A V 2007 Mater. Lett. 61 3292
Caeiro G, Lopes J M, Magnoux P, Ayrault P and Ribeiro F R 2007 J. Catal. 249 234
Carini D J, Duncia J V, Aldrich P E, Chiu A T, Johnson A L, Pierce M E, Price W A, Santella J B, Wells G J, Wexler R R, Wong P C, Yoo S E and Timmermans PBWM 1991 J. Med. Chem. 34 2525
Kohler B, Langer M and Mosandl T 1998 Ger. Pat. Appl. DE19632643C1
Amatore C, Jutand A and Negri S 1990 J. Organomet. Chem. 390 389
Sharp M J and Snieckus V 1985 Tetrahedron Lett. 26 5997
Copar A, Antoncic L and Antoncic M T 2006 Int. Pat. Appl. WO 2006/103068A1
Shashikumar N D, Krishnamurthy G, SundaraRajRao K, Shridhara K, BhojyaNaik H S and Nagarajan K 2010 Org. Process Res. Dev. 14 918
Sharath N, BhojyaNaik H S, VinayKumar B and JoyHoskeri 2011 Brit. J. Pharma. Res. 1 46
(a) Bhimagouda S P, Krishnamurthy G, Bhojyanaik H S, Prashant R L and Manjunath G 2010 Eur. J. Med. Chem. 45 3329; (b) Shashikumar N D 2013 J. Chem. Article ID 240381, 2013 1
Roger J S, Asitha A, Scot C, John L, Mohammed A, Kashem H K, Josephine K, Jennifer A, Kowalski S S, Pullen T, Roma J P, Roth C R, Sarko N S, Wilsyn M P, Winters J P and Wolak C L 2007 Bioorg. Med. Chem. Lett. 17 3660
Ozden O G, Taner E, Hakan G and Sulhiye Y 2007 Bioorg. Med. Chem. Lett. 17 2233
Rohini D S, Alexandar M J N and Chandrasekar 2011 RJPBCS 2 194
Gbolade A A and Adeyemi A A 2008 Fitoterapia 79 223
Acknowledgements
Authors are thankful to the Department of Industrial Chemistry, Kuvempu University, Shimoga, Management and staff of Alkem Laboratories Ltd., R&D center, Bangalore, Karnataka, India, Sri. Venkateshwara Industries and Mandli Industrial Estate, Shimoga for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SHASHIKUMAR, N.D., KRISHNAMURTHY, G., BHOJYANAIK, H.S. et al. Synthesis of new biphenyl-substituted quinoline derivatives, preliminary screening and docking studies. J Chem Sci 126, 205–212 (2014). https://doi.org/10.1007/s12039-013-0541-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0541-4