Abstract
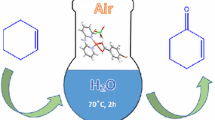
Oxidation of styrene is carried out by using heptamolybdate coordinated transition metal (Co2+, Zn2+) complexes, [2-ampH]4[{Co(H2O)5}Mo7O24] ⋅9H2O (1), [3-ampH]4[{Co(H2O)5}Mo7O24] ⋅9H2O (2), [2-ampH]4[{Zn(H2O)5}Mo7O24] ⋅4H2O (3) and [3-ampH]4[{Zn(3-ampy)(H2O)4}Mo7O24] ⋅4H2O (4) as catalysts and H2O2 as an oxidant at 80°C. The leaching study has been carried out to check the quality of catalyst and it has been reused for three times with good percentage of conversion. For the first two catalysts (compounds 1 and 2), the major product obtained is benzaldehyde, and benzoic acid is the major product for next two catalysts (compounds 3 and 4). Stability of the catalysts has been analyzed by IR, UV-spectroscopy and powder X-ray crystallography.

Catalytic activities of compounds [2-ampH]4[{Co(H2O)5}Mo7O24]·9H2O (1), [3-ampH]4[{Co(H2O)5}Mo7O24]∙9H2O (2), [2-ampH]4[{Zn(H2O)5}Mo7O24]∙4H2O (3) and [3-ampH]4[{Zn(3-ampy)(H2O)4}Mo7O24]∙4H2O (4) have been performed in the conversion of styrene to benzaldehyde/ benzoic acid using H2O2 as an oxidant at a moderate temperature. These catalysts are able to recycle for minimum of three times with similar percentage of conversion.



Similar content being viewed by others
References
(a) Ben-Daniel R, Weiner L and Neumann R 1980 J. Am. Chem.Soc. 124 8788; (b) Hayashi T, Kishida A and Mizuno N 2000 Chemm. Commun. 381; (c) Karcz R, Pamin K, Połtowicz J and Haber J 2009 Catal. Lett. 132 159; (d) Zhao W, Zhang B, Ma Y, Ding Y and Qiu W 2010 Catal. Commun. 11 527; Arumuganthan T, Rao A S, Kumar T V and Das S K 2008 J. Chem. Sci. 120 95
(a) Pope M T 2004 Compr. Coord. Chem. II 4 635; Hill C L 2004 Compr. Coord. Chem. II 4 679; (b) Pope M T and Müller A 1991 Angew. Chem., Int. Ed. 30 34; (c) Hill C L and Prosser-McCartha C M 1995 Coord. Chem. Rev. 143 407; (d) Hill C L 1998 Chem. Rev. 98 1; (e) Izumi Y, Urabe K and Onaka M 1992 In Zeolites, Clay and Heteropolyacid in Organic Reactions (Tokyo: Kodansha); (f) Khan M I, Yohannes E and Doedens R J 2003 Inorg. Chem. 42 3125; (g) Pope M T 1983 In Heteropoly and Isopoly Oxometalates (Berlin: Springer-Verlag)
(a) Yamase T 1993 Mol. Eng. 3 241; (b) Hill C L, Hartnup M, Faraj M, Weeks M, Prosser-McCartha C M,Jr. R B B, Kadkhodayan M, Sommadossi J–P and Schinazi R F 1990 In Advances in Chemotherapy of AIDS, Pharmacology and Therapuetics R B Diasio and J –P Sommadossi (Eds.) (New York: Pergamon)
(a) Pappo R, Jr. D S A, Lemieux R U and Johnson W S J 1956 Org. Chem. 21 478; (b) Carlsen P H J, Katsuki T, Martin V S and Sharpless K B 1981 J. Org. Chem. 46 3936; (c) Michael B S 2002 Organic Synthesis 2nd edition (New York: McGraw- Hill)
Bailey P S 1958 Chem. Rev. 58 925
(a) Sheldon R A and Kochi J K 1981 In Metal-Catalyzed Oxidations of Organic Compounds (New York: Academic Press); (b) Sawyer D T 1991 In Oxygen Chemistry (New York: Oxford University Press)
(a) Jones C W 1999 In Applications of Hydrogen Peroxide and Derivatives (Cambridge: Royal Society of Chemistry); (b) Strukal G (ed.) 1992 In Catalytic Oxidations with Hydrogen Peroxide as Oxidant (Netherlands: Kluwer Academic)
For the international regulations, see: Regulations Concerning the International Carriage of Dangerous Goods by Rail (RID); European Agreement Concerning the International Carriage of Dangerous Goods by Road (ADR); International Maritime Dangerous Goods Code (IMDG Code); International Civil Aviation Organization Technical Instructions for the Safe Transport of Dangerous Goods by Air (ICAOTI); International Air Transport Association Dangerous Goods Regulation (IATA DGR)
Sumitomo Chemical News Release 2000 Oct. 11. Available at http://www.sumitomo-chem.co.jp/english/e1newsrelease/pdf/20001011e.pdf
Dow Products and Businesses News 2002 Aug. 1. Available at http://www.dow.com/dow_news/prodbus/2002/20020801a.htm
(a) Noyori R, Aokib M and Sato K 2003 Chem. Commun. 1977; (b) Neumann R and Gara M 1995 J. Am. Chem. Soc. 117 5066; (c) Xinrong L, Jinyu X, Huizhang L, Bin Y, Songlin J and Gaoyanga X 2000 J. Mol. Catal. A 161 163; (d) Patel K, Tripuramallu B K and Patel A 2011 Eur. J. Inorg. Chem. 1871; (e) Shringapuri P and Patel A 2010 Inorg. Chem. Acta. 362 3796; (f) Patel K, Shringarpuri P and Patel A 2011 Trans. Met. Chem. 36 171
Hill C L 1993 Mol. Eng. 3 263
(a) Li T, Lü J, Gao S and Cao R 2008 Inorg. Chem. Commun. 10 1342; (b) Arumuganthan T, Rao A S and Das S K 2010 Cryst. Growth Des. 10 4272
Sebastian J, Jinka K M and Jasra R V 2006 J. Catal. 244 208
Acknowledgement
We thank Science & Engineering Research Board (a statutory body under the Department of Science and Technology), Government of India, for financial support (Project No. SB/SI/IC/034/2013). We also thank the Centre for Nano Technology, University of Hyderabad. The National X-ray Diffractometer facility at the University of Hyderabad by the Department of Science and Technology, Government of India, is gratefully acknowledged. ASR thanks CSIR, Government of India for a fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
FT-IR spectra, solid state UV spectra, powder X-ray crystallographic data of parent catalysts and used/ regenerated catalysts (compounds 1–4) and the results for the reactions of styrene oxidation with catalysts 1–4 are available in supplementary information at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
AMANCHI, S.R., PATEL, A. & DAS, S.K. Polyoxometalate coordinated transition metal complexes as catalysts: Oxidation of styrene to benzaldehyde/benzoic acid. J Chem Sci 126, 1641–1645 (2014). https://doi.org/10.1007/s12039-014-0719-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0719-4