Abstract
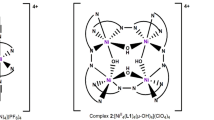
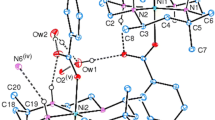
Three dinuclear isophthalato-bridged nickel(II) complexes formulated as [Ni(rac-L)]2(μ-IPA)(ClO4)2 (1), [Ni(RR-L)]2(μ-IPA)(ClO4)2 (2) and [Ni(SS-L)]2(μ-IPA)(ClO4)2 (3) (L = 5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyc-lotetradecane, IPA = isophthalic acid) have been isolated and characterized. Single crystal X-ray diffraction analyses revealed that the Ni(II) atoms have six-coordinated distorted octahedral environments, and the isophthalato ligand bridges two Ni(II) centres in a bis bidentate fashion to form dimers in all three complexes. The monomers of {[Ni(SS-L)]2(μ-IPA)} 2+ are connected through intermolecular hydrogen bonds to generate one-dimensional left-handed helical chains in complex 3. The homochiral natures of complexes 2and3have been confirmed by CD spectroscopy.

Three dinuclear isophthalato-bridged nickel(II) complexes [Ni(rac-L)]2(µ-IPA)(ClO4)2 (1), [Ni(RR-L)]2(µ-IPA)(ClO4)2 (2) and [Ni(SS-L)]2(µ-IPA)(ClO4)2 (3) (L = 5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, IPA = isophthalic acid) were obtained by the reactions of the [Ni(rac-L)]2+/[Ni(RR-L)]2+/[Ni(SS-L)]2+ and isophthalic acid. The homochiral natures of complexes 2 and 3 were confirmed by the results of solid-state circular dichroism spectra measurements.









Similar content being viewed by others
References
Forster P M and Cheetham A K 2002 Angew. Chem. Int. Ed. Engl. 38 457
Ayyappan P, Evans O R and Lin W 2001 Inorg. Chem. 40 4627
Choudhury A, Neeraj S, Natarajan S and Rao C N R 2000 Angew. Chem., Int. Ed. Engl. 39 3091
Braga D, Grepioni F and Desiraju G R 1998 Chem. Rev. 98 1375
Vallejo J, Cano J and Castro I 2012 Chem. Commun. 48 7726
Palacios M A, Mota A J and Ruiz J 2012 Inorg. Chem. 51 7010
Du L Y, Zhang S L and Ding Y Q 2012 Z. Anorg. Allg. Chem. 638 1039
Gao E Q, Zhao Q H, Tang J K, Liao D Z, Jiang Z H and Yan S P 2002 J. Coord. Chem. 55 205
Hong C S and You Y S 2004 Polyhedron 23 1379
Massoud S S, Mautner F A, Vicente R and Rodrigue B M 2006 Inorg. Chim. Acta 359 3321
Ou G C, Yuan X Y and Jiang X P 2011 Trans. Met. Chem. 36 189
Tang J K, Gao E Q, Zhang L, Liao D Z, Jiang Z H and Yan S P 2002 J. Coord. Chem. 55 527
Mautner F A, Louka F R and Massoud S S 2009 J. Mol. Struct. 921 333
Zhao X Y, Liang D D, Liu S X, Sun C Y, Cao R G, Gao C Y, Ren Y H and Su Z M 2008 Inorg. Chem. 47 7133
Mohamadou A, Albada G A, Mutikainen I, Turpeinen U and Reedijk J 2010 Inorg. Chim. Acta 363 3023
Wang Y F, Ju F Y and Wang L Y 2010 Chin. J. Struct. Chem. 29 51
Shin J W, Rowthu S R and Lee J E 2012 Polyhedron 33 25
Sato T, Mori W, Xie Y, Kanehisa N, Kai Y, Fujii M, Goto S, Naga E and Nakao Y 2006 Inorg. Chim. Acta 359 2271
Massoud S S, Mautner F A, Vicente R, Gallo A A and Ducasse E 2007 Eur. J. Inorg. Chem. 1091
Liu X T and Liu Q L 2008 J. Mol. Struct. 889 160
Mahendrasinh Z, Kumar S B, Suresh E and Ribas J 2010 Trans. Met. Chem. 35 757
Yang G M, Li J, Ren Y W, Guo H, Duan M Y, Zhang F X and Zhang X F 2009 Trans. Met. Chem. 34 191
Ou G C, Yuan X Y and Li Z Z 2013 Z. Anorg. Allg. Chem. 639 158
Curtis N F 1960 J. Chem. Soc. (A) 4409
Tait A M and Busch D H 1967 Inorg. Synth. 18 4
Jiang L, Feng X L and Lu T B 2005 Cryst. Growth Des. 5 1469
Sheldrick G M 1996 SADABS Program for Empirical Absorption Correction of Area Detector Data University of Göttingen: Göttingen
Sheldrick G M 2008 Acta Crystallogr. A 64 112
Acknowledgements
This work was supported by the Scientific Research Fund of Hunan Provincial Education Department (13B029, 13A030), the Key Laboratory of Functional Organometallic Materials of Hunan Province College (13K09, 13K10), the Program for Excellent Talents in Hunan University of Science and Engineering (2013), the Construct Program of the Key Discipline in Hunan Province (2011–76), the Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province (2012–318).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Crystallographic data for 1, 2 and 3 have been deposited with the Cambridge Crystallographic Data Centre as supplemental publication numbers CCDC 989829, 989830 and 989831, respectively. Copies of the data can be obtained free of charge via http://www.ccdc.cam.ac.uk.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
OU, GC., LI, ZZ., YUAN, L. et al. Synthesis and crystal structures of three isophthalato-bridged macrocyclic nickel(II) complexes. J Chem Sci 127, 115–122 (2015). https://doi.org/10.1007/s12039-014-0755-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0755-0