Abstract
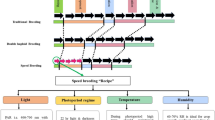
Most traits of interest to medical, agricultural and animal scientists show continuous variation and complex mode of inheritance. DNA-based markers are being deployed to analyse such complex traits, that are known as quantitative trait loci (QTL). In conventional QTL analysis, F2, backcross populations, recombinant inbred lines, backcross inbred lines and double haploids from biparental crosses are commonly used. Introgression lines and near isogenic lines are also being used for QTL analysis. However, such populations have major limitations like predominantly relying on the recombination events taking place in the F1 generation and mapping of only the allelic pairs present in the two parents. The second generation mapping resources like association mapping, nested association mapping and multiparent intercross populations potentially address the major limitations of available mapping resources. The potential of multiparent intercross populations in gene mapping has been discussed here. In such populations both linkage and association analysis can be conductted without encountering the limitations of structured populations. In such populations, larger genetic variation in the germplasm is accessed and various allelic and cytoplasmic interactions are assessed. For all practical purposes, across crop species, use of eight founders and a fixed population of 1000 individuals are most appropriate. Limitations with multiparent intercross populations are that they require longer time and more resource to be generated and they are likely to show extensive segregation for developmental traits, limiting their use in the analysis of complex traits. However, multiparent intercross population resources are likely to bring a paradigm shift towards QTL analysis in plant species.
Similar content being viewed by others
References
Atwell S., Huang Y. S., Vilhjálmsson B. J., Willems G., Horton M., Li Y. et al. 2010 Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465, 627–631.
Bandillo N., Muyco P. A., Caspillo C., Laza M., Sajise A. G., Singh R. K. et al. 2010 Development of multiparent advanced generation intercross (magic) populations for gene discovery in rice (Oryza sativa L.). Philipp. J. Crop Sci. 35, suppl 1, 96.
Bentsink L., Hanson J., Hanhart C. J., Blankestijn-de Vries H., Coltrane C., Keizer P. et al. 2010 Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proc. Natl. Acad. Sci. USA 107, 4264– 4269.
Brachi B., Faure N., Horton M., Flahauw E., Vazquez A., Nordborg M. et al. 2010 Linkage and association mapping of Arabidopsis thaliana flowering time in nature. PLoS Genet. 6, 1–17.
Broman K. W. 2005 The genomes of recombinant inbred lines. Genetics 169, 1133–1146.
Broman K. W., Wu H., Sen S. and Churchill G. A. 2003 R/qtl: QTL mapping in experimental crosses. Bioinformatics 19, 889– 890.
Buckler E. S., Holland J. B., Bradbury P. J., Acharya C. B., Brown P. J., Browne C. et al. 2009 The genetic architecture of maize flowering time. Science 325, 714–718.
Cavanagh C., Morell M., Mackay I. and Powell W. 2008 From mutations to MAGIC: resources for gene discovery, validation and delivery in crop plants. Curr. Opin. Plant Biol. 11, 215– 221.
Churchill G. A., Airey D. C., Allayee H., Angel J. M., Attie A. D., Beatty J. et al. 2004 The collaborative cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 36, 1133–1137.
Collard B. C. Y., Jahufer M. Z. Z., Brouwer J. B. and Pang E. C. K. 2005 An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica 142, 169–196.
Darvasi A. and Soller M. 1995 Advanced intercross lines, an experimental population for fine genetic mapping. Genetics 141, 199–1207.
Darvasi A. and Soller M. 1997 A simple method to calculate resolving power and confidence interval of QTL map location. Behav. Genet. 27, 125–132.
Davey J. W., Hohenlohe P. A., Etter P. D., Boone J. Q., Catchen J. M. and Blaxter M. L. 2011 Genome-wide genetic marker discovery and genotyping using next- generation sequencing. Nat. Rev. Genet. 12, 499–510.
Ehrenreich I. M., Hanzawa Y., Chou L., Roe J. L., Kover P. X. and Purugganan M. D. 2009 Candidate gene association mapping of Arabidopsis flowering time. Genetics 183, 325–335.
Flint J., Valder W., Shifman S. and Mott R. 2005 Strategies for mapping and cloning quantitative trait genes in rodents. Nat. Rev. Genet. 6, 271–286.
Gelderman H. 1975 Investigations on inheritance of quantitative characters in animals by gene markers. Theor. Appl. Genet. 46, 319–330.
Goffinet B. and Gerber S. 2000 Quantitative trait loci: A meta analysis. Genetics 155, 463–473.
Gupta P. K. 2002 Molecular markers and QTL analysis in crop plants. Curr. Sci. 83, 113–114.
Gupta P. K., Rustgi S. and Kulwal P. L. 2005 Linkage disequilibrium and association studies in higher plants: present status and future prospects. Plant Mol. Biol. 57, 461–485.
Gupta P. K., Langridge P. and Mir R. R. 2010 Marker–assisted wheat breeding: present status and future possibilities. Mol. Breed. 26, 145–161.
Hirschhorn J. N. and Daly M. J. 2005 Genome–wide association studies for common diseases and complex traits. Nat. Rev. Genet. 6, 95–108.
Huang B. E. and George A. W. 2011 R/mpMap: a computational platform for the genetic analysis of multiparent recombinant inbred lines. Bioinformatics 27, 727–729.
Huang X., Paulo M. J., Boer M., Effgen S., Keizer P., Koornneef M. and Eeuwijk F. V. 2011 Analysis of natural allelic variation in Arabidopsis using a multiparent recombinant inbred line population. Proc. Natl. Acad. Sci. USA 108, 4488–4493.
Keurentjes J. J., Bentsink L., Alonso-Blanco C., Hanhart C. J., Blankestijn-De Vries H., Effgen S. et al. 2007 Development of a near-isogenic line population of Arabidopsis thaliana and comparison of mapping power with a recombinant inbred line population. Genetics 175, 891–905.
Keurentjes J. J. B., Willems G., van Eeuwijk F., Nordborg M. and Koornneef M. 2011 A comparison of population type used for QTL mapping in Arabidopsis thaliana. Plant Genet. Res. 9, 185– 188.
Kover P. X., Valder W., Trakalo J., Scarcelli N., Ehrenreich I. M., Purugganan M. D. et al. 2009 A multiparent advanced generation inter–cross to fine map quantitative traits in Arabidopsis thaliana. PLoS Genet. 5, e1000551.
Leung H., McNally K., Thomson M., Bandillo N., Muyco P. and Singh R. K. 2011 Expanding and fine-tuning genetic resources for discovery of gene functions in rice. In Plant and animal genome conference, Sandiago (http://www.intl-pag.org/19/abstracts/W80_PAGXIX_500.html).
Li H., Hearne S., Banziger K., Li Z. and Wang J. 2010 Statistical properties of QTL linkage mapping in biparental genetic populations. Heredity 105, 257–267.
Li R., Lyons M. A., Wittenburg H., Paigen B. and Churchill G. A. 2005 Combining data from multiple inbred line crosses improves the power and resolution of quantitative trait loci mapping. Genetics 169, 1699–1709.
Mackay T. F. C. 2004 The genetic architecture of quantitative traits: lessons from Drosophila. Curr. Opin. Genet. Dev. 14, 253– 257.
McMullen M. D., Kresovich S., Villeda H. S., Bradbury P., Li H., Sun Q. et al. 2009 Genetic properties of the maize nested association mapping population. Science 325, 737–740.
Mott R., Talbot C. J., Turri M. G., Collins A. C. and Flint J. 2000 A method for fine mapping quantitative trait loci in outbred animal stocks. Proc. Natl. Acad. Sci. USA 97, 12649–12654.
Nordborg M. and Weigel D. 2008 Next-generation genetics in plants. Nature 456, 720–723.
Rafalski J. A. 2010 Association genetics in crop improvement. Curr. Opin. Plant. Biol. 13, 174–180.
Rakshit S., Zaide P. H. and Mishra S. K. 2002 Molecular markers and tagging of genes in crop plants. In Advances in plant physiology, vol 4 (ed. A. Hemantaranjan), pp. 205–223. Scientific Publications, Jodhpur, India.
Sax K. 1923 Association of size differences with seed–coat pattern and pigmentation in Phaseolus vulgaris. Genetics 8, 552–560.
Sturtevant A. H. 1913 The linear arrangement of six sex-linked factors in Drosophila, as shown by their mode of association. J. Exp. Zool. 14, 43–59.
Valder W., Solberg L. C., Gauguier D., Burnett S., Klenerman P., Cookson W. O. et al. 2006 Genome–wide genetic association of complex traits in heterogeneous stock mice. Nat. Genet. 38, 879–887.
Varshney R. K., Nayak S. N., May G. D. and Jackson S. A. 2009 Next generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 27, 522–530.
Watson J. D. and Crick F. H. C. 1953 Genetical implications of the structure of deoxyribonucleic acid. Nature 171, 964–967.
Yalchin B., Flint J. and Mott R. 2005 Using progenitor strain information to identify quantitative trait nucleotides in out bred mice. Genetics 171, 673–681.
Yu J., Holland J. B., McMullen M. D. and Buckler E. S. 2008 Genetic design and statistical power of nested association mapping in maize. Genetics 178, 539–551.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rakshit S., Rakshit A. and Patil J. V. 2012 Multiparent intercross populations in analysis of quantitative traits. J. Genet. 91, xx–xx
Rights and permissions
About this article
Cite this article
RAKSHIT, S., RAKSHIT, A. & PATIL, J.V. Multiparent intercross populations in analysis of quantitative traits. J Genet 91, 111–117 (2012). https://doi.org/10.1007/s12041-012-0144-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-012-0144-8