Abstract
Purpose
To identify patients with metastatic urothelial cancer (mUC) unlikely to benefit from immune-checkpoint inhibitors (ICIs).
Methods/Patients
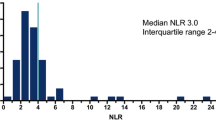
We explored the predictive and prognostic values of baseline neutrophil-to-lymphocyte ratio (NLR), with cut-offs ≥ 3 and ≥ 5, and of a urothelial immune prognostic index (UIPI, based on increased NLR and LDH), on 146 patients.
Results
NLR and UIPI significantly predicted progressive disease and progression-free survival with both cut-offs (p = 0.0069, p = 0.0034, p = 0.0160, p = 0.0063; p < 0.001, p = 0.021, p = 0.014, p = 0.026; for NLR-3, NLR-5, UIPI-3, UIPI-5, respectively) and overall survival when NLR cut-off was ≥ 5 (p = 0.03 and p = 0.024, for NLR-5 and UIPI-5, respectively).
Conclusions
NLR-5 deserves prospective validation to identify mUC patients with poor prognosis following ICIs.

Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386386. https://doi.org/10.1002/ijc.29210.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26. https://doi.org/10.1056/NEJMoa1613683.
Sternberg CN, Loriot Y, James N, Choy E, Castellano D, Lopez-Rios F, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol. 2019;76(1):73–81. https://doi.org/10.1016/j.eururo.2019.03.015.
Hussain SA, Birtle A, Crabb S, Huddart R, Small D, Summerhayes M, et al. From clinical trials to real-life clinical practice: the role of immunotherapy with PD-1/PD-L1 inhibitors in advanced urothelial carcinoma. Eur Urol Oncol. 2018;1(6):486–500. https://doi.org/10.1016/j.euo.2018.05.011.
Tavakkoli M, Wilkins CR, Mones JV, Mauro MJ. A novel paradigm between leukocytosis, G-CSF secretion, neutrophil-to-lymphocyte ratio, myeloid-derived suppressor cells, and prognosis in non-small cell lung cancer. Front Oncol. 2019;9:295. https://doi.org/10.3389/fonc.2019.00295.
Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. https://doi.org/10.1016/j.ctrv.2017.05.005.
Vartolomei MD, Kimura S, Ferro M, Vartolomei L, Foerster B, Abufaraj M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol. 2018;36(7):1019–29. https://doi.org/10.1007/s00345-018-2235-5.
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–7. https://doi.org/10.1001/jamaoncol.2017.4771.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. https://doi.org/10.1016/j.lungcan.2017.01.013.
Li X, Ma X, Tang L, Wang B, Chen L, Zhang F, et al. Prognostic value of neutrophil-to-lymphocyte ratio in urothelial carcinoma of the upper urinary tract and bladder: a systematic review and meta-analysis. Oncotarget. 2017;8(37):62681–92. https://doi.org/10.18632/oncotarget.17467.
Morales Barrera RMI, Gonzalez M, Suarez C, Ros J, Valverde C, Fernandez C, Hierro C, Serra E, Mateo J, Gutierrez S, Martin Liberal J, Quintana A, Dienstmann R, Serrano C, Garralda E, Carles J. Validation of the VIO prognostic index in patients with metastatic urothelial carcinoma treated with immune-checkpoint inhibitors. Ann Oncol. 2019;30:v356–v402 (Suppl. 5).
Acknowledgements
We thank all patients that participated in the study, all involved clinicians and study nurses and the Mediterranean Cancer Support and Rehabilitation (Medicare Onlus) no-profit association for the support to data management.
Funding
None.
Author information
Authors and Affiliations
Contributions
GLB, LM, UDG, UB, GF contributed to study concept and design; RDQ, AA, MM, VU, FR, HL to data acquisition; GLB, LM, UDG, UB to data analysis and interpretation; GLB, RDQ, LM to manuscript drafting; UDG, UB, GF to manuscript critical revision for important intellectual content; GLB, RDQ, GF to statistical analysis; VU, HL to administrative, technical or material support; LM to supervision. All authors contributed to the interpretation of data and to the revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Ethical approval
The authors state that they have obtained appropriate institutional review board approval and have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Informed consent
Informed consent has been obtained from the participants involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12094_2020_2337_MOESM1_ESM.ppt
Supplementary Figure 1. Overall and Progression-Free Survival according to disease response (DCR versus PD): A) OS; B) PFS. (PPT 90 kb)
12094_2020_2337_MOESM2_ESM.ppt
Supplementary Figure 2. Receiver operating characteristic (ROC) curve (A) and dot histogram (B) of NLR based on PD: ROC curve area is 0.67 (95% CI, 0.58-0.75) (p=0.0006), best cut-off 4,6 (sensitivity 0.51, specificity 0.81). (PPT 89 kb)
12094_2020_2337_MOESM3_ESM.ppt
Supplementary Figure 3. Overall and Progression-Free Survival in patients with NLR-5 according to the three Center series, Padua (n=21), Catania (=22), Meldola (n=3): A) OS; Padua (median 3.0; 95% CI, 1.8-4.2), Catania (median 4.8; 95% CI, 3.4-13.0), Meldola (median 3.4; 95% CI, 0.4-7.1) (p=0.658); B) PFS; Padua (median 2.3; 95% CI, 1.0-3.6), Catania (median 2.5; 95% CI, 1.4-3.6), Meldola (median 1.8; 95% CI, 0.3-3.9) (p=0.210). Abbreviations: CR, complete response; DCR, disease control rate (=CR+PR+SD); NLR-3, neutrophils-to-lymphocytes ratio ≥ 3; NLR-5, neutrophils-to-lymphocytes ratio ≥ 5; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; UIPI, urothelial immune prognostic index; UIPI-3, NLR ≥ 3 and lactate dehydrogenase upper the normal value; UIPI-5, NLR ≥ 5 and lactate dehydrogenase upper the normal value. (PPT 101 kb)
Rights and permissions
About this article
Cite this article
Banna, G.L., Di Quattro, R., Malatino, L. et al. Neutrophil-to-lymphocyte ratio and lactate dehydrogenase as biomarkers for urothelial cancer treated with immunotherapy. Clin Transl Oncol 22, 2130–2135 (2020). https://doi.org/10.1007/s12094-020-02337-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02337-3