Abstract
Objectives
To evaluate the correlation between echocardiographic inferior vena cava (IVC) measurements and central venous pressure (CVP) in neonates. Also, to evaluate the correlation between IVC measurements and gestational age (GA) and body weight (BW).
Methods
This cross sectional analytical study was conducted from June 2014 through June 2016 in a level III NICU. All neonates requiring intensive hemodynamic monitoring and having umbilical venous catheter (UVC) in place for clinical indications were enrolled in the study. IVC measurements were recorded by echocardiography (ECHO) and CVP was measured concomitantly in neonates having appropriate sized UVC in place. IVC measurements were evaluated and compared for any correlation with the CVP, GA and BW.
Results
Fifty neonates with median gestation of 37 wk [Q1 = 29.2, Q3 = 37.8, interquartile range (IQR) = 8.6 wk] and median birth weight of 2420 g (Q1 = 923.5, Q3 = 2850, IQR = 1926.5 g) were included in the study. A strong negative linear correlation was observed between IVC collapsibility index (IVC-CI) and CVP (r = −0.968, r2 = −0.937, p 0.000). No correlation was observed between IVC-CI and GA or BW. IVC minimum and IVC maximum diameters did not correlate with CVP but correlated well with GA and BW.
Conclusions
Echocardiographic IVC-CI measurement has a good correlation with CVP measurement in neonates. The clinical use will depend on the ability of IVC-CI to predict surrogate markers of tissue perfusion in shock.
Similar content being viewed by others
Introduction
Cardiac output is determined by the amount of blood returning to the heart (preload), the strength of myocardial contractility and the resistance against which the heart must pump (afterload) [1]. It is therefore, very important to consider preload when choosing treatment strategies for cardiovascular compromise. Despite this, methods for assessing preload in infants are limited. Central venous pressure (CVP) is a good approximation of right atrial pressure,which is a major determinant of right ventricular end diastolic volume. Therefore, CVP is a good indicator of right heart function [2]. CVP has been, and often still is used as a surrogate for preload which in turn helps to define the intravascular fluid volume status and guide fluid management. CVP monitoring is the mainstay of estimating intravascular fluid status and cardiac preload in critically-ill patients [3].
The continuous monitoring of CVP via a catheter is invasive and at times difficult. It is therefore important to devise reliable and less invasive alternatives for use in neonates. The IVC is the biggest vein of venous system with low-pressure. The expansion of the vein reflects venous pressure changes to a certain extent and excess of the intravascular volume. For this reason, the IVC diameter may be an important diagnostic tool in evaluation of hypovolemia and hypervolemia [4]. The diameter of IVC correlates with CVP in adults [5,6,7,8] and children [9] and is used to estimate right heart preload and fluid status. Aim of this study was to evaluate whether echocardiographic measurement of IVC parameters can be used to predict CVP independent of birth weight and gestation.
Material and Methods
This cross sectional analytical study was conducted from 18 June 2014 to 18 June 2016 in the Department of Neonatology, level III NICU, Kanchi Kamakoti CHILDS Trust Hospital, Chennai, India after clearance from the hospital ethical committee. An informed written consent was obtained from the parent or guardian for each neonate enrolled in the study after explaining the study. Based on the sample sizes of previous studies in adult subjects [5, 10], considering probability of type 1 error (α) of 0.05, desired power (β) of 0.8 and to detect a statistical significant correlation of ≥0.5, 50 neonates were needed to be enrolled. All neonates admitted in the NICU within the study period (requiring intensive hemodynamic monitoring having UVC in place for clinical indications as per existing NICU policy) and in sinus rhythm were included. Criteria for exclusion were neonates with congenital heart diseases including mild to severe L-R shunt lesions, valvular lesions, pulmonary hypertension, overt right heart failure, complex cyanotic heart diseases (to avoid influence on right heart and IVC hemodynamics) and neonates on High frequency ventilation (where IVC visualization is difficult). The demographic and basic clinical data was collected in a data collection form to gather a standard data which included patient’s sex, arterial blood pressure (BP) (systolic BP, diastolic BP and mean BP), heart rate (HR), oxygen saturation, status of the patient’s spontaneous or mechanical ventilation and mean airway pressures.
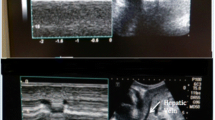
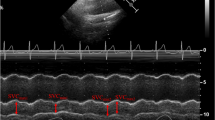
Ultrasonographic measurements of IVC were performed with a portable VIVID-E ultrasound scanner (GE division) with 5 MHz transducer. Measurements of IVC were obtained in the supine position by placing the transthoracic probe sensor subcostally and slowly turning it 90° counter clockwise till an optimal view of IVC entering the right atrium was obtained. After obtaining a 2-D image of the IVC entering the right atrium (Fig. 1), M-mode line was placed through the IVC either close to its entrance to the right atrium or 1 to 2 cm caudal to the hepatic vein–IVC junction (approximately 3–4 cm from the junction of the IVC and the right atrium) and a M-mode tracing was obtained [11]. Thereafter, freezing in M-mode using calipers the maximum and minimum diameter of the IVC tracing were recorded (Fig. 2). Measurements in non-intubated neonates were obtained during their normal spontaneous inspiration and expiration. Ventilated neonates were evaluated during normal ventilator cycling.
IVC collapsibility index (IVC-CI) was measured depending on whether the patient was intubated or not. In non-intubated and ventilated neonates, the IVC-CI was defined as the difference between maximum expiratory diameter and minimum inspiratory diameter divided by the maximum expiratory diameter. The relationship between IVC diameter and respiratory cycle in intubated patients reverses with phases of respiration with maximum diameter being during inspiration due to positive pressure being delivered by mechanical ventilation and minimum diameter during expiration. In the index study, IVC-CI being a ratio was defined as the difference between maximum diameter and minimum diameter divided by the maximum diameter without regard to phases of the respiratory cycle as it was not possible to time inspiration and expiration in neonates. IVC measurements were recorded in millimeters and noted. Central venous pressure (CVP) was measured concomitantly after performing ultrasonographic examination in neonates having appropriate sized UVC in place. The UVC was inserted as high UVC (if it lies above 7th thoracic vertebrae) and the position was verified by X-ray. This is the existing policy of the NICU to confirm the position with X-ray and was not intended for the study purpose. After proper zeroing and calibration, CVP was measured by gravity method by filling the umbilical catheter with one unit per ml of heparinized normal saline solution and then allowing the saline in the catheter to fall, noting the level of column in the umbilical venous catheter. CVP was recorded in cmH2O. Measurements of IVC-CI were then compared with invasively measured CVP. Subsequently, measurements of IVC minimum (IVC min), maximum diameter (IVC max) and IVC-CI were evaluated and compared for any correlation with the corrected gestational age (GA) and birth weight (BW). IVC-CI was also evaluated for any correlation with MAP to see for any influence of positive pressure ventilation on collapsibility index. Categorical measurements were summarized as number and percentage. Numerical measurements like birth weight, gestational age, corrected gestational age, body weight and CVP are expressed as median with interquartile ranges and others as mean with standard deviation (Table 1). Correlations were analyzed by Spearman’s correlation coefficient (r). Linear regressionwas performed to determine coefficient of determination (r2) for variables found to have significant correlations. P value of less than 0.05 were considered to indicate significance in all the statistical analyses, which were performed using SPSS 11.5 version.
Results
Among 50 neonates enrolled, 20 (40%) were girls and 30 (60%) were boys. Forty-seven (94%) neonates were receiving positive pressure ventilation – 4 on CPAP and 43 on mechanical ventilation.
A strong negative correlation was found between IVC-CI and CVP (Fig. 3a: r = −0.968, p 0.000), whereas there was no correlation between IVC max and IVC min measurements with CVP, (Fig. 3b: r = −0.115, p 0.425; Fig. 3c: r = 0.286, p 0.044). Both IVC min and IVC max correlated positively with GA (Fig. 4a: r = 0.759, p 0.000; Fig. 4b: r = 0.859, p 0.000) whereas IVC–CI did not correlate with GA (Fig. 4c: r = 0.111, p 0.442). Both IVC min and IVC max correlated positively with BW (Fig. 5a: r = 0.833, p 0.000; Fig. 5b, r = 0.96, p 0.000) whereas IVC –CI did not correlate with BW (Fig. 5c: r = 0.119, p 0.411). There was also no correlation found between IVC-CI and MAP (r = 0.20, p 0.88). This shows that IVC -CI is independent of both GA and BW and is not influenced by MAP.
Linear regression analysis showed a good strength of linear associations between variables having significant correlations.
IVC-CI and CVP (Fig. 3a), IVC min and IVC max with GA (Fig. 4a, b), IVC min and IVC max with BW (Fig. 5a, b).
Discussion
In this cross-sectional analytical study in neonates, IVC-CI correlated well with central venous pressure but did not correlate with both GA and BW. Thus IVC-CI is not influenced by BW or GA. The present results are similar to the results of adult studies where IVC-CI was found to correlate well with CVP [10, 12].
However the present study demonstrated that IVC min and IVC max both did not correlate with CVP but correlated well with GA and BW. This is in contrast to most adult studies where IVC diameters were found to correlate with CVP [5, 13]. The likely reason is the variation in bodyweight of NICU neonates which is wider than that of adults, the heaviest neonate could weigh eight to ten times more than the lightest one. Sato et al. [14] also reported in their study that IVC diameters (IVC min and IVC max) correlated with GA and BW in neonates.
To add, in adults the correlation between IVC-CI and CVP has been mostly documented in spontaneously breathing patients [15]. In the NICU, infants who require circulatory status assessment often have respiratory disorders requiring mechanical ventilation. Therefore, any method for assessing CVP in infants in the NICU must be applicable to infants under mechanical ventilation. During ventilation with positive end-expiratory pressure (PEEP) support, theoretically it is believed to affect the CVP by increasing the intrathoracic pressure, decreasing venous return and increasing venous stasis, which in turn decreases cardiac output. In fact, PEEP is not transmitted directly to the venous system. In a lung with normal compliance, no more than 25% of the PEEP is transmitted to the central veins. Administration of PEEP leads to compensatory increase in mean systemic pressure at which venous return and cardiac output would be maintained so as the system exists in the equilibrium [16].
In the index study, the values of IVC-CI correlated well with CVP in mechanically ventilated infants. This is likely because PEEP-induced collapse of the inferior vena cava in humans is unlikely due to anatomical reasons. In a study of adults by Schefold [5], patients who were ventilated in a pressure control mode showed significant correlation between expiratory IVC (eIVC) and inspiratory (iIVC) diameter and CVP. Similarly in a study by Stawicki et al. [17], the effect of PEEP on IVC-CI failed to reach a statistical significance. In the index study, the authors did not look for separate subgroup analysis of spontaneously breathing patients as their study had a large percentage of ventilated neonates. The likely reason is that the present study group included sick neonates who required intensive hemodynamic monitoring, had UVC in place and must have required some form of assisted ventilation.
The size of the IVC is influenced by patient position, being smallest in the left lateral position, largest in the right lateral position, and intermediate in the supine position. This may be due to increased intra-abdominal pressure and compression of the IVC by the liver in the left lateral position. In the index study, for consistency and convenience of performing ECHO in sick neonates authors performed all IVC diameter measurements in the supine position.
Ultrasonography is nowadays popularly-used safe, non-invasive and portable tool available in most NICU’s. Accurate measurement of internal structures and also large blood vessels including the IVC, are readily achieved with ultrasound [18].
Studies have shown that bedside ultrasonography can be quickly learned. Hellmann et al. found that internal medicine residents could learn to use hand-held ultrasonography equipment in conventional transthoracic echocardiograms at a “reasonably rapid rate” [19]. DeCara et al. found that it was feasible to teach fourth year medical students to use hand-carried ultrasounds, and that its use also helped in bedside diagnoses [20]. In the index study, the investigator fellow was formally trained in ultrasonography before start of the study. A prior ECHO was done by the pediatric cardiologist to exclude congenital heart diseases. In the index study, the time required to perform bedside evaluation of the inferior vena was approximately 3 min. This trend in using portable ultrasonography as a method of teaching medical students combined with the present integration of this education at the resident level ensures that if IVC measurements can provide for more accurate diagnoses, including that of intravascular status, the next generation of future neonatologists will be well-equipped to use this technology.
The limitations of the index study include: The ultra-sonographic measurements of the IVC were not repeated by another physician or reviewed for precision. As a result, inter-rater reliability was not measured although previous researches have demonstrated good inter-observer agreement in the measurement of IVC as performed by pediatric emergency physicians, even when they were as a group compared to experienced pediatric echocardiography providers [21]. Future studies incorporating multiple measurements taken by separate operators would be useful to assess the inter-observer variability that might exist with this technique. Secondly, the study was not blinded as both IVC and CVP measurements were carried by the same physician who knew about the clinical condition of the baby.
Conclusions
The present study is able to demonstrate a strong correlation between CVP and IVC-CI independent of GA or BW. Clinical use of IVC-CI in neonates needs to be investigated further to predict surrogate markers of tissue perfusion (e.g., lactate, mixed venous oxygen, etc.) and availability of appropriate cut-offs.
References
Taeusch HW, Ballard RA, Gleason CA, Avery ME. Avery’s Diseases of the Newborn. 8th ed. Philadelphia: Elsevier Health Sciences; 2005.
Woods SL, Froelicher ESS, Motzer SU, Bridges EJ. Cardiac Nursing. 6th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
Dalrymple P. Central venous pressure monitoring. Anaes Intens Care Med. 2006;7:91–2.
Gullace G, Savoia MT. Echocardiographic assessment of the inferior vena cava wall motion for studies of right heart dynamics and function. Clin Cardiol. 1984;7:393–404.
Schefold JC, Storm C, Bercker S, et al. Inferior vena cava diameter correlates with invasive hemodynamic measures in mechanically ventilated intensive care unit patients with sepsis. J Emerg Med. 2010;38:632–7.
Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, Dean AJ. The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med. 2011;18:98–101.
Mintz GS, Kotler MN, Parry WR, Iskandrian AS, Kane SA. Real-time inferior vena caval ultrasonography: normal and abnormal findings and its use in assessing right-heart function. Circulation. 1981;64:1018–25.
Krause I, Birk E, Davidovits M, et al. Inferior vena cava diameter: a useful method for estimation of fluid status in children on haemodialysis. Nephrol Dial Transplant. 2001;16:1203–6.
Zengin S, Al B, Genc S, et al. Role of inferior vena cava and right ventricular diameter in assessment of volume status: a comparative study: ultrasound and hypovolemia. Am J Emerg Med. 2013;31:763–7.
Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: a practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thail. 2013;96:S14–22.
Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55:290–5.
Lyon M, Blaivas M, Brannam L. Sonographic measurement of the inferior vena cava as a marker of blood loss. Am J Emerg Med. 2005;23:45–50.
Sato Y, Kawataki M, Hirakawa A, et al. The diameter of the inferior vena cava provides a noninvasive way of calculating central venous pressure in neonates. Acta Paediatr. 2013;102:e241–6.
Natori H, Tamaki S, Kira S. Ultrasonographic evaluation of ventilatory effect on inferior vena caval configuration. Am Rev Respir Dis. 1979;120:421–7.
Luecke T, Pelosi P. Clinical review: positive end-expiratory pressure and cardiac output. Crit Care. 2005;9:607–21.
Stawicki SP, Adkins EJ, Eiferman DS, et al. Prospective evaluation of intravascular volume status in critically ill patients: does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76:956–64.
Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–6.
Hellmann D, Whiting-O’Keefe Q, Shapiro E, Martin L, Martire C, Ziegelstein R. The rate at which residents learn to use hand-held echocardiography at the bedside. Am J Med. 2005;118:1010–8.
DeCara JM, Kirkpatrick JN, Spencer KT, et al. Use of hand-carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses. J Am Soc Echocardiogr. 2005;18:257–63.
Pershad J, Myers S, Plouman C, Rosson C, Elam K, Wan J, Chin T. Bedside limited echocardiography by the emergency physician is accurate during evaluation of the critically ill patient. Pediatrics. 2004;114:e667–71.
Acknowledgements
The authors would like to thank the neonates and their parents for participation in the study.
Author information
Authors and Affiliations
Contributions
MMM: Conception and design, data collection, analysis and interpretation of data; SM Contribution to design of the study and writing the manuscript; RA Analyzing data and editing manuscript. MMM will act as a guarantor for the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Mugloo, M.M., Malik, S. & Akhtar, R. Echocardiographic Inferior Vena Cava Measurement As An Alternative to Central Venous Pressure Measurement in Neonates. Indian J Pediatr 84, 751–756 (2017). https://doi.org/10.1007/s12098-017-2382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2382-5