Abstract
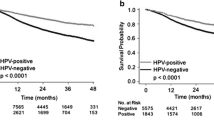
Recent evidence suggests that human papillomavirus (HPV)-positive head and neck squamous cell carcinoma (HNSCC) patients have better survival than HPV-negative patients. However, it is unclear if similar patterns for survival exist across different tumor sites, and whether HPV-associated prognosis is modified by type of treatment. We prospectively tested 222 histologically confirmed HNSCC primary tumors for HPV DNA by PCR and HPV E6/E7 RNA by RT-PCR prior to treatment at a large urban health center. Cox proportional hazard ratio models were constructed to assess HPV-associated differences in overall and disease-specific survival adjusting for clinical and demographic covariates. HPV detection varied significantly by primary HNSCC tumor site, from 35 % for oropharynx, to 25 % for hypopharynx, 5 % for larynx, and 3 % for oral cavity (p < 0.0001), with HPV16 accounting for the majority (95 %) of HPV-positive tumors. The hazard-risk of overall and disease-specific death comparing HPV16-positive versus negative oropharyngeal HNSCC was reduced by 74 and 89 %, respectively (p values < 0.05), and was independent of other prognostic indicators; no statistically significant changes in outcomes were observed for non-oropharyngeal HNSCC sites. Prediction of overall survival was better with combined DNA and RNA HPV16 positive PCR detection. There was no difference in HPV16-associated survival whether patients received either surgery or (chemo)radiotherapy as their initial treatment modality. Improved HPV-associated HNSCC survival is limited to patients with oropharyngeal primaries. No selective treatment advantage is observed for HPV-positive tumors, although clinical trials are needed to evaluate which treatment modalities provide the most benefit for HPV-positive HNSCC.



Similar content being viewed by others
References
Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–20. doi:10.1093/jnci/djn025.
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9. doi:10.1093/jnci/djn011.
Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–20. doi:10.1002/ijc.22851.
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20.
Lace MJ, Anson JR, Klussmann JP, Wang DH, Smith EM, Haugen TH, et al. Human papillomavirus type 16 (HPV-16) genomes integrated in head and neck cancers and in HPV-16-immortalized human keratinocyte clones express chimeric virus-cell mRNAs similar to those found in cervical cancers. J Virol. 2011;85(4):1645–54. doi:10.1128/JVI.02093-10.
Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5–8. Int J Cancer. 2003;107(3):394–400. doi:10.1002/ijc.11389.
Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(Suppl 1):S59–66. doi:10.1016/j.jcv.2004.11.017.
Morshed K. Association between human papillomavirus infection and laryngeal squamous cell carcinoma. J Med Virol. 2010;82(6):1017–23. doi:10.1002/jmv.21749.
Kim KM, Cho NH, Choi HS, Kim YH, Byeon HK, Min HJ, et al. Effect of human papilloma virus expression on clinical course of laryngeal papilloma. Acta Otolaryngol. 2008;128(10):1138–44. doi:10.1080/00016480701827509.
Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology Inc. 2011. doi:10.1038/modpathol.2011.91.
Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24(36):5630–6. doi:10.1200/JCO.2005.04.6136.
Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(12):1992–8. doi:10.1200/JCO.2008.20.2853.
Kumar B, Cordell KG, Lee JS, Prince ME, Tran HH, Wolf GT, et al. Response to therapy and outcomes in oropharyngeal cancer are associated with biomarkers including human papillomavirus, epidermal growth factor receptor, gender, and smoking. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S109–11. doi:10.1016/j.ijrobp.2007.05.072.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi:10.1056/NEJMoa0912217.
Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68(3):417–23. doi:10.1002/jmv.10220.
Schiffman M, Rodriguez AC, Chen Z, Wacholder S, Herrero R, Hildesheim A, et al. A population-based prospective study of carcinogenic human papillomavirus variant lineages, viral persistence, and cervical neoplasia. Cancer Res. 2010;70(8):3159–69. doi:10.1158/0008-5472.
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10(4):321–2.
el Awady MK, Kaplan JB, O’Brien SJ, Burk RD. Molecular analysis of integrated human papillomavirus 16 sequences in the cervical cancer cell line SiHa. Virology. 1987;159(2):389–98.
Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, Leemans CR, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004;96(13):998–1006.
van Houten VM, Snijders PJ, van den Brekel MW, Kummer JA, Meijer CJ, van Leeuwen B, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93(2):232–5. doi:10.1002/ijc.1313.
Michiels S, Le Maitre A, Buyse M, Burzykowski T, Maillard E, Bogaerts J, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: meta-analyses of individual patient data. Lancet Oncol. 2009;10(4):341–50. doi:10.1016/S1470-2045(09)70023-3.
Greenland S, Robins JM. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol. 1986;15(3):413–9.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–55.
Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178–83.
Hosmer D, Lemeshow S. Applied survival analysis. New York: Wiley; 1999.
O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–201. doi:10.1016/j.oraloncology.2012.06.019.
Rose Ragin CC, Taioli E. Second primary head and neck tumor risk in patients with cervical cancer–SEER data analysis. Head Neck. 2008;30(1):58–66. doi:10.1002/hed20663.
Reddy VM, Cundall-Curry D, Bridger M. Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985–2006. Ann R Coll Surg Engl. 2010;92(8):655–9. doi:10.1308/003588410X12699663904871.
Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103(9):1843–9. doi:10.1002/cncr.20998.
Robinson KL, Macfarlane GJ. Oropharyngeal cancer incidence and mortality in Scotland: are rates still increasing? Oral Oncol. 2003;39(1):31–6.
Attner P, Du J, Nasman A, Hammarstedt L, Ramqvist T, Lindholm J, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer. 2010;126(12):2879–84. doi:10.1002/ijc.24994.
Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125(2):362–6. doi:10.1002/ijc.24339.
Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9. doi:10.1200/JCO.2007.14.1713.
Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–35. doi:10.1002/cncr.22963.
Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119(11):2620–3. doi:10.1002/ijc.22177.
Klaes R, Woerner SM, Ridder R, Wentzensen N, Duerst M, Schneider A, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59(24):6132–6.
Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. doi:10.1038nrc798.
Shi W, Kato H, Perez-Ordonez B, Pintilie M, Huang S, Hui A, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–21. doi:10.1200/JCO.2009.23.1670.
Bristow RG, Benchimol S, Hill RP. The p53 gene as a modifier of intrinsic radiosensitivity: implications for radiotherapy. Radiother Oncol. 1996;40(3):197–223.
Mellin H, Dahlgren L, Munck-Wikland E, Lindholm J, Rabbani H, Kalantari M, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102(2):152–8. doi:10.1002/ijc.10669.
Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–47. doi:10.1200/JCO.2004.00.3335.
Smith EM, Wang D, Kim Y, Rubenstein LM, Lee JH, Haugen TH, et al. P16INK4a expression, human papillomavirus, and survival in head and neck cancer. Oral Oncol. 2008;44(2):133–42. doi:10.1016/j.oraloncology.2007.01.010.
Soria JC, Rodriguez M, Liu DD, Lee JJ, Hong WK, Mao L. Aberrant promoter methylation of multiple genes in bronchial brush samples from former cigarette smokers. Cancer Res. 2002;62(2):351–5.
Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126(5):1256–62. doi:10.1002/ijc.24842.
Sethi S, Ali-Fehmi R, Franceschi S, Struijk L, van Doorn LJ, Quint W, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer. 2012;131(5):1179–86. doi:10.1002/ijc.26500.
Liang C, Marsit CJ, McClean MD, Nelson HH, Christensen BC, Haddad RI, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72(19):5004–13. doi:10.1158/0008-5472.CAN-11-3277.
Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Stat Med. 2007;26(16):3078–94. doi:10.1002/sim.2781.
D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81. doi:10.1002/(SICI)1097-0258(19981015)17:19<2265:AID-SIM918>3.0.CO;2-B.
Rosenbaum PR. Discussing hidden bias in observational studies. Ann Intern Med. 1991;115(11):901–5.
Phaeton R, Wang XG, Einstein MH, Goldberg GL, Casadevall A, Dadachova E. The influence of proteasome inhibitor MG132, external radiation, and unlabeled antibody on the tumor uptake and biodistribution of (188)re-labeled anti-E6 C1P5 antibody in cervical cancer in mice. Cancer. 2010;116(4 Suppl):1067–74. doi:10.1002/cncr.24794.
Acknowledgments
We thank the participants of this study; Margaret Brandwein-Gensler for her help with pathological staging and processing of tissue specimens; Catherine Sarta for her time and effort spent enrolling participants and with data entry; Gregory Rosenblatt for his assistance with data management; and Leslie Adrien for her help preparing and handling the biospecimens for molecular analysis. This work is supported in part by the National Cancer Institute [grant number CA115243]; National Institute of Dental and Craniofacial Research [T32 training grant DE007255]; the Einstein Cancer Research Center (P30 grant CA013330); and the Departments of Otorhinolaryngology-Head and Neck Surgery and Pathology at Albert Einstein College of Medicine and Montefiore Medical Center.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salazar, C.R., Smith, R.V., Garg, M.K. et al. Human Papillomavirus-Associated Head and Neck Squamous Cell Carcinoma Survival: A Comparison by Tumor Site and Initial Treatment. Head and Neck Pathol 8, 77–87 (2014). https://doi.org/10.1007/s12105-013-0486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-013-0486-4