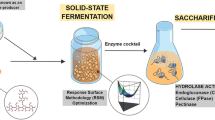
Abstract
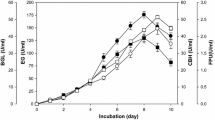
Efficient, low-cost enzymatic hydrolysis of lignocellulosic biomass is essential for cost-effective production of bioethanol. The aim of this study was to establish a fungal fermentation-based strategy for the economic enzymatic conversion of pineapple peel into fermentable sugars. Trichoderma viride was grown on passion fruit peel in order to improve its β-glucosidase production, and a crude extract was then used to hydrolyze pineapple peel. The effects of medium pH, cultivation time, and passion fruit peel concentration on β-glucosidase production were evaluated using a central composite rotational design (CCRD) combined with response surface methodology (RSM). Optimal β-glucosidase activity of 2.40 U mL−1 was found after 6.5 days of cultivation in medium at pH 6.0, containing 2.0 % passion fruit peel. Saccharification of pineapple peel was also optimized by RSM and CCRD with respect to pH, temperature, β-glucosidase concentration, and reaction time and proceeded optimally at pH 4.0, 55 °C, with a β-glucosidase loading of 31.25 U g−1 dry feedstock and 75 h of reaction. Under these conditions, T. viride crude extract hydrolyzed pineapple peel with a glucose yield of 65.3 %. This study therefore presents passion fruit peel as an attractive raw material for the production of β-glucosidases. In addition, it describes an improved, effective, and low-cost enzymatic method for the production of fermentable sugars from pineapple peel, an abundant and inexpensive agro-industrial waste.


Similar content being viewed by others
References
Sanchéz C (2009) Lignocellulosic residues: biodegradation and bioconversion fungi. Biotechnol Adv 27(2):185–194
Bhatia L, Johri S, Ahmad R (2012) An economic and ecological perspective of ethanol production from renewable agro waste: a review. ABM Express 2(1):2–19
Sethi S, Datta AP, Gupta BL, Gupta S (2013) Optimization of cellulose production from bacteria isolated from soil. ISRN Biotechnol 2013 Article ID 985685.
Dashtban M, Schraft H, Qin W (2009) Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int J Biol Sci 5(6):578–595
Singhania RR, Parameswaran B, Pandey A (2009) Handbook of plant-based biofuels. In: Pandey A (ed) Plant-based biofuels: an introduction. CRC Press, Boca Raton, pp 3–12
Sindhu R, Kuttiraja M, Binod P, Janu KU, Sukumaran RK, Pandey A (2011) Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresour Technol 102(23):10915–10921
Dhillon GS, Oberoi HS, Kaur S, Bansal S, Brar SK (2011) Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind Crops Prod 34(1):1160–1167
Adsul MG, Singhvi MS, Gaikaiwari SA, Gokhale DV (2011) Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour Technol 102(6):4304–4312
Zilly A, Bazanella GCS, Helm CV, Araujo CAV, Souza CGM, Bracht A, Peralta RM (2012) Solid-state bioconversion of passion fruit waste by white-rot fungi for production of oxidative and hydrolytic enzymes. Food Bioprocess Technol 5(5):1573–1580
Brazilian Institute of Geography and Statistics (IBGE) (2012). Municipal Agricultural Production - Temporary and Permanent Crops 2012. Rio de Janeiro (Brazil): IBGE. ftp://ftp.ibge.gov.br /Producao_Agricola/Producao_Agricola_Municipal_[anual]/2013/pam2013.pdf. Accessed 05 June 2015
Pinheiro ER, Silva IMDA, Gonzaga LV, Amante ER, Teófilo RF, Ferreira MMC, Amboni RDMC (2008) Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour Technol 99(13):5561–5566
Pavan FA, Lima EC, Dias SLP, Mazzocato AC (2008) Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J Hazard Mater 150(3):703–712
Rani DS, Nand K (2004) Ensilage of pineapple processing waste for methane generation. Waste Manag 24(5):5523–5528
FAOSTAT (2013) Food and Agriculture Organization. Agriculture statistics. Pineapple production (2010) by country. http://faostat.fao.org/. Accessed 10 June 2015
Isitua CC, Ibeh IN (2010) Novel method of wine production from banana (Musa acuminata) and pineapple (Ananas comosus) wastes. Afr J Biotechnol 9(44):7521–7524
Upadhyay A, Lama JP, Tawata S (2010) Utilization of pineapple waste: a review. J Food Sci Technol 6:10–18
Beitel SM, Knob A (2013) Penicillium miczynskii β-glucosidase: a glucose-tolerant enzyme produced using pineapple peel as substrate. Ind Biotechnol 9(5):293–300
Fortkamp D, Knob A (2014) High xylanase production by Trichoderma viride using pineapple peel as substrate and its application in pulp bleaching. Afr J Biotechnol 13(22):2248–2259
Nigan JN (2000) Continuous ethanol production from pineapple cannery waste using immobilized yeast cells. J Biotechnol 80(2):189–193
Hossain ABMS, Fazliny AR (2010) Creation of alternative energy by bio-ethanol production from pineapple waste and the usage of its properties for engine. Afr J Microbiol Res 4(9):813–819
Niwaswong C, Patiwat C, Chotikosaikanon P, Ruangviriyachai C (2014) Simple and enhanced production of lignocellulosic ethanol by diluted acid hydrolysis process of pineapple peel (Ananas comosus) waste. Afr J Biotechnol 13(38):3928–3934
Choonut A, Saejong M, Sangkharak K (2014) The production of ethanol and hydrogen from pineapple peel by Saccharomyces cerevisiae and Enterobacter aerogenes. Energy Procedia 52:242–249
Pirota R, Delabona P, Farinas C (2014) Enzymatic hydrolysis of sugarcane bagasse using enzyme extract and whole solid-state fermentation medium of two newly isolated strains of Aspegillus oryzae. Chem Eng Trans 38:259–264
Visser EM, Leal TF, Almeida MN (2015) Guimarães VM (2015) Increased enzymatic hydrolysis of sugarcane bagasse from enzyme recycling. Biotechnol Biofuels 8:5
Zimbardi ALRL, Sehn C, Meleiro LP, Souza FHM, Masui DC, Nozawa MSF, Guimarães LHS, Jorge JA, Furriel RPM (2013) Optimization of β-glucosidase, β-xylosidase and xylanase production by Colletotrichum graminicola under solid-state fermentation and application in raw sugarcane trash saccharification. Int J Mol Sci 14(2):2875–2902
Vogel HJ (1956) A convenient growth medium for (medium N) Neurospora crassa. Microb Genet Bull 13:42–43
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Kersters-Hilderson H, Claeyssens M, Doorslaer EV, Sna E, Bruyne CK (1982) β-D-Xylosidase from Bacillus pumilus. Methods Enzymol 83:631–639
Muthuvelayudham R, Viruthagiri T (2006) Fermentative production and kinetics of cellulase protein on Trichoderma reesei using sugarcane bagasse and rice straw. Afr J Biotechnol 5(20):1873–1881
Yang B, Wyman CE (2004) Effect of xylan and lignin removal by batch and flow through pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol Bioeng 86(1):88–95
Vallander L, Eriksoon K-E (1985) Enzymic saccharification of pretreated wheat straw. Biotechnol Bioeng 27:650–659
Chen M, Qin Y, Liu Z, Liu K, Wang F, Qu Y (2010) Isolation and characterization of a β-glucosidase from Penicillium decumbens and improving hydrolysis of corncob residue by using it as cellulase supplementation. Enzyme Microb Technol 46(6):444–449
Meyer AS, Rosgaard L, Sørensen HR (2009) The minimal enzyme cocktail concept for biomass processing. J Cereal Sci 50(3):337–344
El-Naggar NE, Adbelwahed AM, Saber WIA, Mohamed AA (2014) Bioprocessing of some agro-industrial residues for endoglucanase production by the new subsp. Streptomyces albogriseolus subsp. cellulolyticus strain NEAE-J. Braz J Microbiol 45(2):743–756
Akinyele JB, Olaniyi OO (2013) Investigation of the cellulases production by Aspergillus niger NSPR 002 in different cultivation conditions. Innov Rom Food Biotechnol 13:71–79
Irshad M, Anwar Z, Ramzan M, Mahmood Z, Nawaz H (2013) Characterization of purified β-glucosidase produced from Trichoderma viride through bio-processing of orange peel waste. Adv Biosci Biotechnol 4:941–944
Almeida JM, Lima VA, Giloni-Lima PC, Knob A (2015) Passion fruit peel as novel substrate for enhanced β-glucosidases production by Penicillium verruculosum: potential of the crude extract for biomass hydrolysis. Biomass Bioenergy 72:216–226
Jalis H, Ahmad A, Khan SA, Sohail M (2014) Utilization of apple peels for the production of plant cell-wall degrading enzymes by Aspergillus fumigatus MS16. J Anim Plant Sci 24(2):64–67
Silva CAA, Lacerda MPF, Leite RSR, Fonseca GG (2013) Production of enzymes from Lichtheimia ramosa using Brazilian savannah fruit wastes as substrate on solid-state bioprocesses. Electron J Biotechnol 16(5):1–9
Lee YM, Lee H, Kim JS, Lee J, Ahn BJ, Kim G-H, Lee J, Ahn BY, Kim G-H, Kim J-J (2014) Optimization of medium components for β-glucosidase production in Schizophyllum commune KUC9397 and enzymatic hydrolysis of lignocellulosic biomass. Bioresour 9(3):4358–4368
Masui DC, Zimbardi AL, Souza FH, Guimarães LH, Furriel RP, Jorge JA (2012) Production of a xylose-stimulated β-glucosidase and a cellulase-free thermostable xylanase by the thermophilic fungus Humicola brevis var. thermoidea under solid state fermentation. World J Microbiol Biotechnol 28(8):2689–2701
Baig MMV (2005) Cellulolytic enzymes of Trichoderma lignorum produced on banana agro-waste: optimization of culture medium and conditions. J Sci Ind Res 64:57–60
Sørensen A, Andersen JJ, Ahring BK, Teller PJ, Lübeck M (2014) Screening of carbon sources for beta-glucosidase production by Aspergillus saccharolyticus. Int Biodet Biodegrad 93:78–83
Ouyang J, Li X, Ying H, Yong Q (2009) Enhanced enzymatic conversion and glucose production via two-step enzymatic hydrolysis of corn cob residue from xylooligosaccharides producer’s waste. Bioresour 4(4):1586–1599
Moreira LRS, Ferreira GV, Ferreira-Filho EXF (2012) The hydrolysis of agro-industrial residues by holocellulose-degrading enzymes. Braz J Microbiol 43:498–505
Saha BC, Cotta MA (2008) Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenergy 32:971–977
Saini JK, Anurag RK, Arya A, Kumbhar BK, Tewari L (2013) Optimization of saccharification of sweet sorghum bagasse using response surface methodology. Ind Crops Prod 44:211–219
El-Tabey TS, Abdelhafez AA, Ali SH, Ramadan EM (2012) Effect of acid hydrolysis and fungal biotreatment on agro-industrial wastes for obtainment of free sugars for bioethanol production. Braz J Microbiol 43(4):1523–1535
Pensupa N, Jin M, Kokolski M, Archer DB, Du CA (2013) Solid state fungal fermentation-based strategy for the hydrolysis of wheat straw. Bioresour Technol 149:261–267
Qing Q, Yang B, Wyman CE (2010) Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour Technol 101:9624–9630
Maeda RN, Serpa VI, Rocha VAL, Mesquita RAA, Anna LMMS, Castro AM, Driemeier CE, Pereira-JR N, Polikarpov I (2011) Enzymatic hydrolysis of pretreated sugar cane bagasse using Penicillium funiculosum and Trichoderma harzianum cellulases. Process Biochem 46(5):1196–1201
Delabona PS, Cota J, Hoffmam ZB, Paixao DA, Farinas CS, Cairo JP, Lima DJ, Squina FM, Ruller R, Pradella JG (2013) Understanding the cellulolytic system of Trichoderma harzianum P49P11 and enhancing saccharification of pretreated sugarcane bagasse by supplementation with pectinase and α-L-arabinofuranosidase. Bioresour Technol 131:500–507
El-Zawawy WK, Ibrahim MM, Abdel-Fattah YR, Soliman NA, Mahmoud MM (2011) Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production. Carbohydr Polym 84(3):865–871
Yang B, Dai Z, Ding S-Y, Wyman CE (2011) Enzymatic hydrolysis of cellulosic biomass. Biofuels 2(4):421–450
Acknowledgments
The authors would like to thank the National Counsel of Technological and Scientific Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support and the scholarship awarded to the first author, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Almeida, J.M., de Lima, V.A., de Lima, P.C.G. et al. Effective and Low-Cost Saccharification of Pineapple Peel by Trichoderma viride Crude Extract with Enhanced β-Glucosidase Activity. Bioenerg. Res. 9, 701–710 (2016). https://doi.org/10.1007/s12155-016-9714-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-016-9714-6