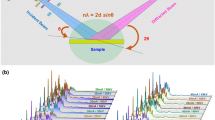
Abstract
Potassium bromate is used in the baking industry as a bread improver and is known to improve dough kneading characteristics and bread volume. However, over the years, its carcinogenic nature has been established and KBrO3 is considered a hazardous adulterant in food systems. This study aimed to develop and validate a novel powder x-ray diffraction (PXRD) method for the detection of KBrO3 adulteration in bakery ingredients and products. Different combinations of x-ray tube voltage (25–45 kV), tube current (25–45 mA), step size (0.006°–0.13°), and time per step (50–150 s) were experimented on an angle of 15°–90° at 25 °C with CuKα radiation (λ = 1.5418 Å). The KBrO3 phase was identified based on the peak values of 2θ and Miller indicate (hkl) and was confirmed using ICCD and COD crystallography databases. Based on the observation, x-ray tube voltage (45 kV), current (45 mA), step size (0.006 Å), and net time per step (150.450 s) were found to be the optimal diffraction conditions for the detection of KBrO3. This study demonstrates the potential of XRD as a non-destructive, rapid, and reliable method for the detection of KBrO3 in bakery products.






Similar content being viewed by others
References
Aggrawal M, Rohrer JS (2020) Selective and sensitive determination of bromate in bread by ion chromatography-mass spectrometry. J Chromatogr A 1615:460765
DeWitt KM, Batson J, Witkowski M, Ranieri N, Richards-Waugh L (2015) X-ray powder diffraction method development and validation for the identification of counterfeit pharmaceuticals. Mater Sci 1–28
Guo W et al (2016) Sensitive screening of bromate in drinking water by an improved ion chromatography ICP-MS method. Microchem J 124:127–131
Hay DG, Jaeger H, West GW (1985) Examination of the monoclinic/orthorhombic transition in silicalite using XRD and silicon NMR. J Phys Chem 89(7):1070–1072
Jenkins R, Nichols M (1989) Problems in the derivation of d-values from experimental digital XRD patterns. Adv X-ray Anal 33:285–293
Korschelt K et al (2018) CeO 2− x nanorods with intrinsic urease-like activity. Nanoscale 10(27):13074–13082
Lee J-W et al (2020) A deep-learning technique for phase identification in multiphase inorganic compounds using synthetic XRD powder patterns. Nat Commun 11(1):1–11
Link AN et al (2009) National Institute of Standards and Technology. Gov Entrep (October):87–101. https://doi.org/10.1093/acprof:oso/9780195369458.003.0005
Matta S et al (2017) Phosphoric acid production by attacking phosphate rock with recycled hexafluosilicic acid. Int J Miner Process 161:21–27
Mendenhall MH, Mullen K, Cline JP (2015) An implementation of the fundamental parameters approach for analysis of X-ray powder diffraction line profiles. J Res Natl Inst Stand Technol 120:223–251. https://doi.org/10.6028/jres.120.014
Pal M, Devrani M, Pintoo S (2018) Significance of hygienic processing of milk and dairy products. Madridge J Food Technol 3:133–137
Reddy TJR, Achari VBS, Sharma AK, Rao VN (2007) Effect of plasticizer on electrical conductivity and cell parameters of (PVC+KBrO3) polymer electrolyte system. Ionics 13(1):55–59
Santamaria-Perez D et al (2015) Crystal behavior of potassium bromate under compression. Acta Crystallogr Sect B: Struct Sci Cryst Eng Mater 71(6):798–804
Shanmugavel V et al (2020) Potassium bromate: effects on bread components, health, environment and method of analysis: a review. Food Chem 311:125964
Shoba US, Udupa MR (1994) The thermal decomposition of potassium bromate in the presence of chromium (III) oxide. Thermochim Acta 242:215–221
Suksaeree J et al (2014) Physicochemical properties study of Plai patches for topical applications. Int J Pharm Pharm Sci 6(5):253–256
Walfish S (2006) Analytical methods: a statistical perspective on the ICH Q2A and Q2B guidelines for validation of analytical methods. BioPharm Int 19(12):1–6
Yokota A et al (2012) Sensitive and simple determination of bromate in foods disinfected with hypochlorite reagents using high performance liquid chromatography with post-column derivatization. J Chromatogr A 1262:219–222
Zakaria P, Bloomfield C, Shellie RA, Haddad PR, Dicinoski GW (2011) Determination of bromate in sea water using multi-dimensional matrix-elimination ion chromatography. J Chromatogr A 1218(50):9080–9085. https://doi.org/10.1016/j.chroma.2011.10.029
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals.
Informed Consent
Informed consent not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 2569 kb)
Rights and permissions
About this article
Cite this article
Paranthaman, R., Moses, J.A. & Anandharamakrishnan, C. A Powder X-Ray Diffraction Method for Qualitative Detection of Potassium Bromate in Bakery Ingredients and Products. Food Anal. Methods 14, 1054–1063 (2021). https://doi.org/10.1007/s12161-020-01943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01943-9