Abstract
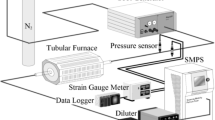
Understanding the mechanism of carbon oxidation is important for the successful modeling of diesel particulate filter regeneration. Characteristics of soot oxidation were investigated with carbon black (Printex-U). A flow reactor system that could simulate the condition of a diesel particulate filter and diesel exhaust gas was designed. Kinetic constants were derived and the reaction mechanisms were proposed using the experimental results and a simple reaction scheme, which approximated the overall oxidation process in TPO as well as CTO. From the experiments, the apparent activation energy for carbon oxidation with NO2-O2-H2O was determined to be 40±2 kJ/mol, with the first order of carbon in the range of 10∼90% oxidation and a temperature range of 250∼500°C. This value was exceedingly lower than the activation energy of NO2-O2 oxidation, which was 60±3 kJ/mol. When NO2 exists with O2 and H2O, the reaction rate increases in proportion to NO2. It increases nonlinearly with O2 or H2O concentration when the other two oxidants are fixed.
Similar content being viewed by others
References
Allansson, R., Blakeman, P. G., Cooper, B. J., Hess, H., Silcock, P. J. and Walker, A. P. (2002). Optimising the low temperature performance and regeneration efficiency of the continuously regenerating diesel particulate filter (CR-DPF) system. SAE Paper No. 2002-01-0428.
Bokova, M. N., Decarne, C., Abi-Aad, E., Pryakhin, A. N., Lunin, V. V. and Aboukais, A. (2005). Kinetics of catalytic carbon black oxidation. Thermochimica ACTA, 428,1/2, 165–171.
Clague, A. D. H., Donnet, J. B., Wang, T. K. and Peng, J. C. M. (1999). A comparison of diesel engine soot with carbon black. Carbon, 37, 1553–1565.
Gilot, P., Nonnefoy, F., Marcuccilli, F. and Prado, G. (1993). Determination of kinetic data for soot oxidation. Modeling of competition between oxygen diffusion and reaction during thermogravimetric analysis. Combustion and Flame, 95, 87–100.
Haralampous, O. A., Kandylas, I. P., Koltsakis, G. C. and Samaras, Z. C. (2004). Experimental evaluation of apparent soot oxidation rates in diesel particulate filters. Int. J. Vehicle Design 35,4, 365–382.
Jacquot, F., Logie, V., Brilhac, J. F. and Gilot, P. (2002). Kinetics of the oxidation of carbon black by NO2 influence of the presence of water and oxygen. Carbon, 40, 335–343.
Jung, H., Kittelson, D. B. and Zachariah, M. R. (2004). Kinetics and visualization of soot oxidation using transmission electron microscopy. Combustion and Flame, 136, 445–456.
Lee, H. S. and Chun, K. M. (2006). Investigation of soot oxidation characteristics in a simulated diesel particulate filter. Int. J. Automotive Technology 7,3, 261–267.
Lee, J. H., Lee, H. S., Song, S. H. and Chun, K. M. (2007). Experimental investigation of soot oxidation characteristic with NO2 and O2 using a flow reactor simulating DPF. SAE Paper No. 2007-01-1270.
Neeft, J. P. A., Hoornaert, F., Makkee, M. and Moulijn, J. A. (1996). The effects of heat and mass transfer in thermogravimetrical analysis. A case study towards the catalytic oxidation of soot. Thermochimica ACTA, 261–278.
Su, D. S., Jentoft, R. E., Muller, J.-O., Rothe, D., Jacob, E., Sompson, C. D., Tomovic, Z., Mullen, K., Messerer, A., Poschal, U., Niessner, R. and Schologl, R. (2004). Microstructure and oxidation behaviour of Euro IV diesel engine soot: A comparative study with synthetic model soot substances. Catalysis Today, 90, 127–132.
Stratakis, G. A. and Stamatelos, A. M. (2003). Thermo-gravimetric analysis of soot emitted by a modern diesel engine run on catalyst-doped fuel. Combustion and Flame, 132, 157–169.
Yezerets, A., Currier, N. W., Eadler, H., Popuri, H. and Suresh, A. (2002). Quantitative flow-reactor study of diesel soot oxidation process. SAE Paper No. 2002-01-1684.
Yezerets, A., Currier, N. W., Eadler, H. A., Suresh, A., Madden, P. F. and Branigin, M. A. (2003). Investigation of the oxidation behavior of diesel particulate matter. Catalysis Today, 88, 17–25.
Vander Wal, R. L. and Tomasek, A. J. (2003). Soot oxidation: Dependence upon initial nasostructure. Combustion and Flame, 134, 1–9.
Vincent, M. W., Rechards, P. J. and Rogers, T. J. (2002). Effective particulates reduction in diesel engines through the use of fuel catalysed particulate filters. Int. J. Automotive Technology 3,1, 1–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, J., Lee, J.H., Song, S. et al. Measurement of soot oxidation with No2-O2-H2O in a flow reactor simulating diesel engine DPF. Int.J Automot. Technol. 9, 423–428 (2008). https://doi.org/10.1007/s12239-008-0051-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12239-008-0051-4