Abstract
Purpose
The objective of this work was to develop a new nanostructured lipid carrier (NLC) formulation for the oral delivery of quetiapine fumarate (QTF) and assess the drug’s in vitro release mechanism through gastric and intestinal conditions.
Methods
A preformulation study was conducted to select the most suitable components and solid-to-liquid lipid ratio for the formulation of nanoparticles. Then, a central composite design was employed to optimize the development of NLC and to study the effect of lipid and surfactant percentages on the physical characteristics of the preparation. The optimal formulation was subjected to physicochemical characterization and stability study. An in vitro release assay using simulated gastrointestinal fluids was performed to study the QTF release mechanism.
Results
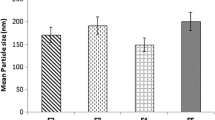
The optimal formulation showed good particle size, PDI, and zeta potential of 179.2 ± 2.6 nm, 0.220 ± 0.020, and −33.63 ± 0.23 mV, respectively. The encapsulation efficiency and the loading capacity were 84.49 ± 1.25% and 2.6 ± 0.03%, respectively. DSC and FTIR analysis showed compatibility between QTF and other components of the formulation and successful encapsulation of the drug within lipid nanoparticles. The optimal formulation also showed good long-term stability at 4 °C storage temperature. The in vitro release of QTF followed the Korsmeyer-Peppas model. The study demonstrated that QTF was mainly released by diffusion mechanism in the gastric medium, and by erosion and anomalous transport in the intestinal medium.
Conclusion
NLC represents a suitable formulation for the oral delivery of QTF. Further studies should investigate the oral absorption and lymphatic transport potential of the optimized formulation.







Similar content being viewed by others
References
DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine. Clin Pharmacokinet. 2001;40(7):509–22. https://doi.org/10.2165/00003088-200140070-00003.
Sanford M, Keating GM. Quetiapine. CNS Drugs. 2012;26(5):435–60. https://doi.org/10.2165/11203840-000000000-00000.
Cheer SM, Wagstaff AJ. Quetiapine. CNS Drugs. 2004;18(3):173–99. https://doi.org/10.2165/00023210-200418030-00004.
Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. https://doi.org/10.1016/S0939-6411(00)00087-4.
Rainer HM, Ranjita S, Cornelia MK. 20 years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr Drug Discov Technol. 2011;8(3):207–27. https://doi.org/10.2174/157016311796799062.
Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine: Nanotech Bio Med. 2016;12(1):143–61. doi:https://doi.org/10.1016/j.nano.2015.09.004.
Beloqui A, del Pozo-Rodríguez A, Isla A, Rodríguez-Gascón A, Solinís MÁ. Nanostructured lipid carriers as oral delivery systems for poorly soluble drugs. Journal of Drug Delivery Science and Technology. 2017;42:144–54. https://doi.org/10.1016/j.jddst.2017.06.013.
Ganesan P, Narayanasamy D. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Cities Soc. 2017;6:37–56. https://doi.org/10.1016/j.scp.2017.07.002.
Poonia N, Kharb R, Lather V, Pandita D. Nanostructured lipid carriers: versatile oral delivery vehicle. Future Science OA. 2016;2(3):FSO135. https://doi.org/10.4155/fsoa-2016-0030.
Lawless E, Griffin BT, O’Mahony A, O’Driscoll CM. Exploring the impact of drug properties on the extent of intestinal lymphatic transport - in vitro and in vivo studies. Pharm Res. 2015;32(5):1817–29. https://doi.org/10.1007/s11095-014-1578-x.
Chaturvedi S, Garg A, Verma A. Nano lipid based carriers for lymphatic voyage of anti-cancer drugs: an insight into the in-vitro, ex-vivo, in-situ and in-vivo study models. J Drug Delivery Sci Technol. 2020;59: 101899. https://doi.org/10.1016/j.jddst.2020.101899.
Pandya P, Giram P, Bhole RP, Chang HI, Raut SY. Nanocarriers based oral lymphatic drug targeting: strategic bioavailability enhancement approaches. J Drug Delivery Sci Technol. 2021:102585. https://doi.org/10.1016/j.jddst.2021.102585.
Poovi G, Damodharan N. Lipid nanoparticles: a challenging approach for oral delivery of BCS Class-II drugs. Future J Pharm Sci. 2018;4(2):191–205. https://doi.org/10.1016/j.fjps.2018.04.001.
Fang G, Tang B, Chao Y, Zhang Y, Xu H, Tang X. Improved oral bioavailability of docetaxel by nanostructured lipid carriers: in vitro characteristics, in vivo evaluation and intestinal transport studies. RSC Adv. 2015;5(117):96437–47. https://doi.org/10.1039/C5RA14588K.
Rangaraj N, Pailla SR, Shah S, Prajapati S, Sampathi S. QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: evaluation using chylomicron flow blocking approach. Drug Deliv Transl Res. 2020;10(5):1476–94. https://doi.org/10.1007/s13346-020-00803-7.
Shrivastava S, Gidwani B, Kaur CD. Development of mebendazole loaded nanostructured lipid carriers for lymphatic targeting: optimization, characterization, in-vitro and in-vivo evaluation. Part Sci Technol. 2021;39(3):380–90. https://doi.org/10.1080/02726351.2020.1750515.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33. https://doi.org/10.1016/S0928-0987(01)00095-1.
Faidi A, Lassoued MA, Becheikh MEH, Touati M, Stumbé JF, Farhat F. Application of sodium alginate extracted from a Tunisian brown algae Padina pavonica for essential oil encapsulation: microspheres preparation, characterization and in vitro release study. Int J Biol Macromol. 2019;136:386–94. https://doi.org/10.1016/j.ijbiomac.2019.06.023.
Patel N, Baldaniya M, Raval M, Sheth N. Formulation and development of in situ nasal gelling systems for quetiapine fumarate-loaded mucoadhesive microemulsion. J Pharm Innov. 2015;10(4):357–73.
Moghddam SMM, Ahad A, Aqil M, Imam SS, Sultana Y. Optimization of nanostructured lipid carriers for topical delivery of nimesulide using Box-Behnken design approach. Artificial Cells, Nanomedicine, and Biotechnology. 2017;45(3):617–24. https://doi.org/10.3109/21691401.2016.1167699.
Tapeinos C, Battaglini M, Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release. 2017;264:306–32. https://doi.org/10.1016/j.jconrel.2017.08.033.
Pinto F, de Barros DPC, Reis C, Fonseca LP. Optimization of nanostructured lipid carriers loaded with retinoids by central composite design. J Mol Liq. 2019;293: 111468. https://doi.org/10.1016/j.molliq.2019.111468.
Hejri A, Khosravi A, Gharanjig K, Hejazi M. Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013;141(1):117–23. https://doi.org/10.1016/j.foodchem.2013.02.080.
Zhang J, Fan Y, Smith E. Experimental design for the optimization of lipid nanoparticles. J Pharm Sci. 2009;98(5):1813–9. https://doi.org/10.1002/jps.21549.
Vitorino C, Carvalho FA, Almeida AJ, Sousa JJ, Pais AACC. The size of solid lipid nanoparticles: an interpretation from experimental design. Colloids Surf, B. 2011;84(1):117–30. https://doi.org/10.1016/j.colsurfb.2010.12.024.
Fathi HA, Allam A, Elsabahy M, Fetih G, El-Badry M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf, B. 2018;162:236–45. https://doi.org/10.1016/j.colsurfb.2017.11.064.
Gaba B, Fazil M, Ali A, Baboota S, Sahni JK, Ali J. Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration. Drug Delivery. 2015;22(6):691–700. https://doi.org/10.3109/10717544.2014.898110.
Tan SW, Billa N, Roberts CR, Burley JC. Surfactant effects on the physical characteristics of Amphotericin B-containing nanostructured lipid carriers. Colloids Surf, A. 2010;372(1):73–9. https://doi.org/10.1016/j.colsurfa.2010.09.030.
Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69(1):1–9. https://doi.org/10.1016/j.ejpb.2007.08.001.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57.
Chuacharoen T, Sabliov CM. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf, A. 2016;503:11–8. https://doi.org/10.1016/j.colsurfa.2016.04.038.
Witayaudom P, Klinkesorn U. Effect of surfactant concentration and solidification temperature on the characteristics and stability of nanostructured lipid carrier (NLC) prepared from rambutan (Nephelium lappaceum L.) kernel fat. J Colloid Interface Sci. 2017;505:1082–92. https://doi.org/10.1016/j.jcis.2017.07.008.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLNTM) dispersions. Int J Pharm. 1998;168(2):221–9. https://doi.org/10.1016/S0378-5173(98)00092-1.
Rydhag L, Wilton I. The function of phospholipids of soybean lecithin in emulsions. J Am Oil Chem Soc. 1981;58(8):830–7. https://doi.org/10.1007/BF02665591.
Wang G, Wang T. Oxidative stability of egg and soy lecithin as affected by transition metal ions and pH in emulsion. J Agric Food Chem. 2008;56(23):11424–31. https://doi.org/10.1021/jf8022832.
Han F, Li S, Yin R, Liu H, Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Colloids Surf, A. 2008;315(1):210–6. https://doi.org/10.1016/j.colsurfa.2007.08.005.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. https://doi.org/10.1208/s12249-010-9563-0.
Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 2012;20(10):813–30. https://doi.org/10.3109/1061186X.2012.716845.
Muchow M, Maincent P, Muller RH. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm. 2008;34(12):1394–405. https://doi.org/10.1080/03639040802130061.
Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1):121–8. https://doi.org/10.1016/S0378-5173(02)00180-1.
Katouzian I, Faridi Esfanjani A, Jafari SM, Akhavan S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci Technol. 2017;68:14–25. https://doi.org/10.1016/j.tifs.2017.07.017.
Shah B, Khunt D, Misra M, Padh H. Application of Box-Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route*. Int J Biol Macromol. 2016;89:206–18. https://doi.org/10.1016/j.ijbiomac.2016.04.076.
Gohel MC, Patel TM. Compatibility study of quetiapine fumarate with widely used sustained release excipients. J Therm Anal Calorim. 2013;111(3):2103–8. https://doi.org/10.1007/s10973-012-2467-3.
Hernández Y, Lozano T, Morales-Cepeda AB, Navarro-Pardo F, Ángeles ME, Morales-Zamudio L, et al. Stearic acid as interface modifier and lubricant agent of the system: Polypropylene/calcium carbonate nanoparticles. Polym Eng Sci. 2019;59(s2):E279–85. https://doi.org/10.1002/pen.25053.
Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomed Nanotechnol Biol Med. 2009;5(2):184–91. https://doi.org/10.1016/j.nano.2008.08.003.
Das S, Ng WK, Tan RBH. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47(1):139–51. https://doi.org/10.1016/j.ejps.2012.05.010.
Agarwal S, HariKumar SL, Negi P, Upadhyay N, Garg R. Quetiapine fumarate loaded nanostructured lipid carrier for enhancing oral bioavailability: design, development and pharmacokinetic assessment. Curr Drug Deliv. 2021;18(2):184–98. https://doi.org/10.2174/1567201817999200728135119.
Narala A, Veerabrahma K. Preparation, characterization and evaluation of quetiapine fumarate solid lipid nanoparticles to improve the oral bioavailability. J Pharm. 2013;2013: 265741. https://doi.org/10.1155/2013/265741.
Son GH, Lee BJ, Cho CW. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J Pharm Investig. 2017;47(4):287–96. https://doi.org/10.1007/s40005-017-0320-1.
Porter CJH, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60(6):673–91. https://doi.org/10.1016/j.addr.2007.10.014.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discovery. 2007;6(3):231–48. https://doi.org/10.1038/nrd2197.
Zhuang CY, Li N, Wang M, Zhang XN, Pan WS, Peng JJ, et al. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm. 2010;394(1):179–85. https://doi.org/10.1016/j.ijpharm.2010.05.005.
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–44. https://doi.org/10.1016/j.jconrel.2008.10.002.
Costa FO, Sousa JJS, Pais AACC, Formosinho SJ. Comparison of dissolution profiles of Ibuprofen pellets. J Control Release. 2003;89(2):199–212. https://doi.org/10.1016/S0168-3659(03)00033-6.
Öztürk AA, Aygül A, Şenel B. Influence of glyceryl behenate, tripalmitin and stearic acid on the properties of clarithromycin incorporated solid lipid nanoparticles (SLNs): formulation, characterization, antibacterial activity and cytotoxicity. J Drug Delivery Sci Technol. 2019;54: 101240. https://doi.org/10.1016/j.jddst.2019.101240.
Rehman M, Ihsan A, Madni A, Bajwa SZ, Shi D, Webster TJ, et al. Solid lipid nanoparticles for thermoresponsive targeting: evidence from spectrophotometry, electrochemical, and cytotoxicity studies. Int J Nanomedicine. 2017;12:8325–36. https://doi.org/10.2147/IJN.S147506.
Acknowledgements
The authors acknowledge Professor Hatem Fessi from the laboratory LAGEP-UMR 5007 (Claude Bernard University Lyon 1, France) for his help in TEM analysis. The authors also acknowledge Professor Salette Reis and Cláudia Nunes from the laboratory REQUIMTE (Faculdade de Farmácia, Universidade do Porto, Portugal) for providing the simulated intestinal fluids powder and for their help with FTIR analysis.
Author information
Authors and Affiliations
Contributions
O.B.H.A., M.A.L., and S.S. conceived and designed the experiment. O.B.H.A. performed the experimental work. O.B.H.A. and M.A.L. analyzed the experimental results. O.B.H.A. and M.A.L. wrote the paper. All the authors reviewed the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ben Hadj Ayed, O., Lassoued, M. & Sfar, S. Quality-by-Design Approach Development, Characterization, and In Vitro Release Mechanism Elucidation of Nanostructured Lipid Carriers for Quetiapine Fumarate Oral Delivery. J Pharm Innov 17, 840–855 (2022). https://doi.org/10.1007/s12247-021-09567-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09567-0