Abstract
To understand the epidemiological and clinical features of the symptomatic and asymptomatic pediatric cases of COVID-19, we carried out a prospective study in Shanghai during the period of January 19 to April 30, 2020. A total of 49 children (mean age 11.5 ± 5.12 years) confirmed with SARS-CoV-2 infection were enrolled in the study, including 11 (22.4%) domestic cases and 38 (77.6%) imported cases. Nine (81.8%) local cases and 12 (31.6%) imported cases had a definitive epidemiological exposure. Twenty-eight (57.1%) were symptomatic and 21 (42.9%) were asymptomatic. Neither asymptomatic nor symptomatic cases progressed to severe diseases. The mean duration of viral shedding for SARS-CoV-2 in upper respiratory tract was 14.1 ± 6.4 days in asymptomatic cases and 14.8 ± 8.4 days in symptomatic cases (P > 0.05). Forty-five (91.8%) cases had viral RNA detected in stool. The mean duration of viral shedding in stool was 28.1 ± 13.3 days in asymptomatic cases and 30.8 ± 18.6 days in symptomatic participants (P > 0.05). Children < 7 years shed viral RNA in stool for a longer duration than school-aged children (P < 0.05). Forty-three (87.8%) cases had seropositivity for antibodies against SARS-CoV-2 within 1–3 weeks after confirmation with infection. In conclusion, asymptomatic SARS-CoV-2 infection may be common in children in the community during the COVID-19 pandemic wave. Asymptomatic cases shed viral RNA in a similar pattern as symptomatic cases do. It is of particular concern that asymptomatic individuals are potentially seed transmission of SARS-CoV-2 and pose a challenge to disease control.
Similar content being viewed by others
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sparked the national outbreak of coronavirus disease 2019 (COVID-19) in China’s mainland during January to February 2020 (WHO 2020c). The disease spread quickly to the world and World Health Organization (WHO) characterized COVID-19 as a pandemic on 11 March 2020 (WHO 2020a). The ongoing COVID-19 pandemic has imposed a huge burden on the health systems and caused widespread social and economic disruption in the world. With the SARS-CoV-2 outbreaks rapidly intensifying worldwide, the importation of COVID-19 cases arrived in China in early March 2020 (National Health Commission of The People’s Republic of China 2020). A total of 339 local COVID-19 cases including 11 (3.3%) children and 313 imported COVID-19 cases including 38 (12.1%) children were reported in Shanghai as of 30 April 2020 (Shanghai Municipal Health Commission 2020).
Children are generally vulnerable to respiratory virus infections. However, the reported cases of COVID-19 and severe patients mostly occurred in adults while pediatric COVID-19 accounted for only 1.7%–6.2% of the total cases based on the initial surveillance data from China, Korea, and the US (CDC COVID-19 Response Team 2020; Choi et al. 2020; WHO 2020c). The attack rate of COVID-19 in children seems to be low but this does not necessarily mean they are less susceptible. SARS-CoV-2 is highly contagious with an estimated R0 being 2.2–5.7 among Wuhan population before interventions (Li et al. 2020; Sanche et al. 2020; Zhang et al. 2020). Thus, all age groups are theoretically at the similar risk of COVID-19 without robust public health interventions. A contact-based surveillance in Shenzhen found that infection rate in children was comparable with or slightly higher than in younger adults (Bi et al. 2020). Due to the different screening strategies of COVID-19 in Shanghai during the domestic and the global wave, we noticed the complexity of SARS-CoV-2 infection in children. Herein, we described the clinical and epidemiological features of all pediatric cases of COVID-19 in Shanghai, the largest metropolitan city in China during January 19 to April 30, 2020, aiming to add our knowledge and understanding of SARS-CoV-2 infection in children.
Materials and Methods
Data Collection
Clinical and epidemiological data were collected from 49 laboratory-confirmed pediatric cases with SARS-CoV-2 infection in Shanghai between January and April in 2020, who were admitted to a tertiary children’s hospital designated for management of pediatric COVID-19 patients. The data on epidemiology, clinical symptoms and course, laboratory findings, chest imaging and treatment regimen were obtained via a face-to-face interview with parents or teenagers and electronical medical chart.
To monitor viral shedding, repeated nasopharyngeal and oropharyngeal swabs and stool or rectal swabs were collected from all confirmed cases every three days until the nucleic acids of SARS-CoV-2 were undetectable. Urine and blood were also collected to detect the nucleic acids of SARS-CoV-2 within 24 h after admission. According to the national protocol (National Health Commission and State Administration of Chinese Traditional Medicine 2020), all confirmed cases were mandatorily isolated at hospital until 2 consecutive respiratory samples (collected at least 24 h apart) test negative for the nucleic acids of SARS-CoV-2. All cases were followed up for 2 weeks after hospital discharge.
Criteria of Screening SARS-CoV-2 Infection
All local patients were screened for SARS-CoV-2 if they had acute fever or respiratory illness and had at least one of the followings during the 14 days prior to symptom onset: a) contact with a confirmed or suspect COVID-19 case; b) a history of travel to or residence in Wuhan or other areas reporting community transmission of COVID-19 disease. Prior to 31 March, symptom-based screening strategy was implemented for all international travelers. Since 31 March, all international travelers were screened for SARS-CoV-2, irrespective of clinical symptoms and signs.
Laboratory Protocol for the Detection of SARS-CoV-2
SARS-CoV-2 infection was laboratory-confirmed by real-time RT-PCR assay, based on the positive test for the nucleic acid of SARS-CoV-2 with two respiratory samples consecutively collected at least 24 h apart. The China CDC specific primers and probes for the detection of SARS-CoV-2 were designed to target both open reading frame 1ab gene and nucleocapsid protein gene (WHO 2020b). A commercial kit (Shanghai bio-germ Medical Technology Co Ltd, China) was used for testing at Shanghai CDC laboratory and the cycle threshold value < 38 was defined as a positive test. To monitor the viral RNA shedding in respiratory samples and stool, nasopharyngeal swab, pharyngeal swab and stool specimen were taken sequentially from all confirmed cases for the detection of SARS-CoV-2 RNA every three days until the nucleic acids of SARS-CoV-2 were undetectable in 2 consecutive samples taken 24 h apart.
Detection of Serum IgM and IgG for SARS-CoV-2
Serum SARS-CoV-2 specific IgG and IgM combined antibodies were tested for all confirmed cases. Paired serum was collected at admission, 7–14 days after confirmation with symptomatic and asymptomatic infection and on discharged. The IgG and IgM combined Antibody test Kit based on immunochromatography method (Guangzhou Wondfo Biotech Co Ltd, China) were used. The antibodies target the S protein of SARS-CoV-2. When the SARS-CoV-2 antibodies level in the specimen is at or above the target cutoff (the detection limit of the test), the antibodies bound to the antigen-dye conjugate are captured by anti-human IgG antibody and anti-human μ chain antibody immobilized in the test region.
Statistical Analysis
Data were analyzed using STATA version 10.0 (Stata Corp, College Station, TX, USA). The measurement data was analyzed by one-way ANOVA, the Scheffe’s post hoc test was used to compare the difference among the groups. The categorical data was analyzed by Chi square test or Fisher’s exact test. A difference with P < 0.05 was considered to be significant.
Results
Demographics of the Confirmed Pediatric Cases
Of the 49 confirmed pediatric cases, 11 (22.4%) were domestic cases including Shanghai residents (8) children and Wuhan residents (3), and 38 (77.6%) were imported cases from European countries (28), the US (8), Canada (1) and Philippines (1). The ratio of male-to female was 1.3 (28/21); the mean age was 11.5 ± 5.12 years with 9 (18.4%) aged 7 months–6 years, 16 (32.7%) aged 7–12 years and 24 (48.9%) aged 13–17 years. Domestic cases were significantly younger than imported cases (5.8 ± 4.1 years vs. 13.2 ± 4.1 years, P < 0.05).
Epidemiological Exposure
Twenty-one (42.9%) children had contact with confirmed or suspect symptomatic cases of COVID-19. Exposure settings included household in 15 cases (71.4%), school dormitory in 5 cases (23.8%), and travel bus in 1 (4.8%) case. Of the 11 domestic cases, 9 (81.8%) had a clear epidemiological linkage with 8 confirmed family cluster cases and 1 traveler cases. Of the 38 imported cases, 12 (31.6%) had a clear contact with 7 confirmed or suspect family cluster cases and 5 school-dormitory cases.
Clinical Manifestations and Course
On initial screening, 28 (57.1%) cases were symptomatic and 21 (42.9%) cases were asymptomatic. Chest CT and X-ray were performed for 40 cases and 9 cases, respectively. Lung lesions were revealed in 20 (40.8%) cases including 15 symptomatic cases and 5 asymptomatic cases. The prominent radiographic findings included ground-glass opacity and patchy infiltrates. Neither the 13 mild cases nor the 16 asymptomatic carriers developed radiographic evidence of pneumonia during follow-up investigation. None of the cases progressed to severe disease.
Twenty-one (42.9%) had cough, 16 (32.7%) had fever, 8 (16.3%) had sore throat, 8 (16.3%) had stuffy nose, 7 (14.2%) had rhinorrhea (Table 1). Six (12.2%) children aged 15–17 years including 1 asymptomatic carrier and 5 symptomatic cases self-reported transient loss of taste and smell during hospitalization. Of the 16 febrile patients, 12 (24.5%) had a body temperature of 37.5 °C–38.4 °C and 4 (8.2%) had a body temperature of 38.5 °C–40.0 °C; the duration of fever ranged from 1 to 8 days (mean: 2.1 ± 1.9 days, median: 1 day). Antiviral drugs were not recommended for treatment. Hydroxychloroquine was administered to 5 cases > 14 years for 1–2 days and then discontinued considering that the patients’ conditions were mild and stable. A 17-year-old patient received hydroxychloroquine on day 7 after disease onset because he had persistent 7-day fever and increased lung lesions revealed by chest CT, and fever subsided 1 day after hydroxychloroquine treatment. One patient with pneumonia received 3-day oral azithromycin therapy because he was confirmed with mycoplasma pneumoniae infection.
Laboratory Findings
The laboratory findings in symptomatic cases and asymptomatic cases were shown in Table 2. All cases showed a normal range of serum biochemistry markers on admission and during follow-up testing except one patient who developed mild liver injury (alanine aminotransferase/aspartate aminotransferase: 110/59 IU/L) after 6-day hydroxychloroquine therapy. The elevated liver enzyme returned to the normal range after discontinuation of hydroxychloroquine. Leukopenia (the counts of white blood cell < 4.0 × 109/L) was seen in 5 cases, neutropenia (the counts of neutrophil < 0.5 × 109/L) was seen 2 asymptomatic cases with radiographic evidence of pneumonia. One mild case showed an elevated level of C-reactive protein (CRP) (35 mg/L). There were no statistically significant differences in the serum biochemistry markers, CRP, procalcitonin and other laboratory tests between the symptomatic group and asymptomatic group.
Viral RNA Shedding and Serum Specific Antibody Testing
None of the 49 cases had SARS-CoV-2 RNA detected in serum and urine. Forty-five (91.8%) cases had SARS-CoV-2 RNA detected in stool. The patterns of viral shedding were shown in Fig. 1. Overall, the duration of viral RNA shedding is longer in gastrointestinal tract than in upper respiratory tract (P < 0.05). There was no significant difference in the mean duration of viral shedding between asymptomatic and symptomatic cases in upper respiratory tract (14.1 ± 6.4 days vs. 14.8 ± 8.4 days, P > 0.05) and in gastrointestinal tract (28.1 ± 13.3 days vs. 30.8 ± 18.6 days, P > 0.05). Young children < 7 years of age shed viral RNA in stool for a significantly longer duration than school-aged children ≥ 7 years of age (P < 0.05, Table 3).
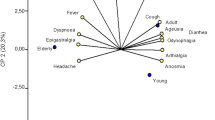
Forty-three (87.8%) of the 49 cases including 25 symptomatic cases and 18 asymptomatic cases had seropositivity for IgG and IgM combined antibodies against SARS-CoV-2 within 1–3 weeks after confirmation with asymptomatic infection or disease onset. As shown in Fig. 2, 19 (44.2%) cases developed seropositivity for antibodies within 1 week, 14 (32.6%) cases developed seropositivity for antibodies within 1–2 weeks, and 10 (23.3%) cases developed seropositivity for antibodies within 2–3 weeks. We noted that 66.7% of asymptomatic cases developed seropositivity within 1 week while only 28% of symptomatic cases developed seropositivity within 1 week. Among 6 cases who had seronegative, the last serum sample tested for antibodies was collected on day 8–22 after disease onset or confirmation with infection before hospital discharge.
The patterns of antibody response to SARS-CoV-2 by clinical symptomatology (A) and age (B). The figure shows the percentage of the antibody production time in different stage including 66.7% of asymptomatic cases developed seropositivity within 1 week, only 28% of symptomatic cases developed seropositivity within 1 week.
Discussion
The current global spread of SARS-CoV-2 infection requires our evolving knowledge about the epidemiology and clinical findings of COVID-19. Results from the universal entry screening of international travelers irrespective of symptoms in Shanghai reveal that asymptomatic SARS-CoV-2 infection maybe common in children during community transmission in the global pandemic wave. Early testing has largely been limited to symptomatic individuals fulfilling the criteria of suspect cases in China, the US and European countries (National Health Commission of The People’s Republic of China; Centers for Disease Control and Prevention 2020; Tagarro et al. 2020) which may miss individuals who were not identified as being at a risk of COVID-19. In this study, we observed three important aspects in asymptomatic pediatric cases: first, none of asymptomatic pediatric cases in this study progressed to symptomatic cases; second, the mean duration of viral shedding was similar in symptomatic and asymptomatic cases, which likely indicates asymptomatic cases could have the same transmissibility as asymptomatic cases; third, asymptomatic cases had earlier seropositivity for antibodies than symptomatic cases did, which suggests asymptomatic cases had acquired SARS-CoV-2 infection for some time before laboratory-confirmation and mandatory isolation. It has been shown that asymptomatic individuals caused secondary spread of SARS-CoV-2 (Hu et al. 2020; Rothe et al. 2020). Thus, of particular concern is that asymptomatic individuals are usually active in the community and potentially seed transmission of SARS-CoV-2 in population.
In China, 68%–90% of domestic pediatric cases had a clear epidemiological linkage to family cluster cases (Cai et al. 2020; Lu et al. 2020; Qiu et al. 2020; Wang et al. 2020). In this study, 81.8% of local cases were linked to transmission chain, predominantly in household setting, while only 28.9% of imported pediatric cases acquired infection through close contact with suspect or confirmed cases in family or school dormitory. The distinct transmission pattern suggests sustained SARS-CoV-2 transmission has occurred in the community settings in the imported countries. So far, the community prevalence of SARS-CoV-2 infection among children is unclear. In fact, pediatric cases of COVID-19 not linkable to transmission chains in Wuhan experiencing large outbreaks of local transmission were largely missed because 90.1% of confirmed cases were from family cluster cases (Lu et al. 2020). Additionally, our data along with Wuhan’s report showed that local cases were significantly younger than imported cases. This phenomenon could also reflect the distinct transmission patterns of COVID-19 between young children and old children. We also observed a few of imported cases linkable to school exposure. It is noteworthy that school closures largely reduce the risk of SARS-CoV-2 infection in children. In China school closure has shown effect on reducing peak incidence by 40%–60% (Zhang et al. 2020). In the long term, school closures would be unlikely to prevent the epidemic of COVID-19 among children unless vaccines are available for use.
Children with COVID-19 disease usually had a brief fever with a mean duration of 2.5 days and the spike of fever was usually not higher than 39 °C. Our previous studies showed that children with influenza had the longer duration of fever (mean: 4 days) and 67% of children had a fever ≥ 39.0 °C (Ge et al. 2012; Wang et al. 2015). The characteristics of fever are helpful to clinically distinguish COVID-19 from influenza. In this study, non-severe COVID-19 disease in children was almost self-limited without a specific treatment. To date, no evidence has shown effectiveness of any antiviral agent and hydroxychloroquine in the treatment of COVID-19 (Bhimraj et al. 2020). Thus, antiviral therapy should be cautiously recommended for children with COVID-19. Based on our clinical evaluation, imaging investigation should not be routinely recommended for individuals with asymptomatic COVID-19 or symptomatic disease without clinical signs of disease progression.
The mean duration of viral RNA shedding in upper respiratory tract was much longer in SARS-CoV-2 infection than in influenza virus infections (14.5 days verse 3.4–5.2 days) (Loeb et al. 2012). This finding could suggest the higher potential of COVID-19 in its transmissibility than that of influenza. Besides, we found that 91.8% of children with COVID-19 shed virus in stool, also the duration of viral shedding is much longer in gastrointestinal tract than in upper respiratory tract, especially in younger children. Live SARS-CoV-2 has been isolated in stool from COVID-19 patients (Yong et al. 2020). However, the impact on SARS-CoV-2 shedding in feces on the transmission model remains unclear. Further research is needed to incorporate these viral shedding data into an analysis of the transmission of SARS-CoV-2, to infer the relationship between the duration and quantity of viral shedding and infectivity and secondary transmission events.
Currently RT-PCR is a gold standard for the COVID-19 diagnosis recommended by WHO (Tang et al. 2020), but point-of-care rapid diagnostic tests (POCT) for SARS-CoV-2 antibodies are useful for the field screening. The reliability of POCT is influenced by the diagnostic kits and the timing of serum sampling. Our study showed that 87.3% of children with COVID-19 developed IgM/IgG combined antibodies within 1–3 weeks after symptom onset or infection. At least immunochromatography-based rapid antibody test can be used as an alternative diagnostic method for screening COVID-19. As the majority of patients develop seropositivity for antibodies, clinical explanation of the results should fully consider the timing of testing.
The strength of this study is that we followed up the entire clinical course, dynamic antibody seroconversion and viral shedding in symptomatic and asymptomatic pediatric cases with confirmed COVID-19. One limitation is that we couldn’t obtain the viral load to quantify the dynamic levels of viral shedding over the disease course to determine the effect of the quantity of viral shedding on the duration of viral shedding and the clinical symptomatology. Another limitation is that we couldn’t trace the close contacts of confirmed pediatric cases to investigate the rate of secondary infection. Further research is required to fully understand the natural history of COVID-19 infection in children and answer remained questions on the community prevalence of SARS-CoV-2 infection among children and the contribution of persons with asymptomatic COVID-19 to influenza virus transmission in household, institutional, and community settings.
References
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ, O’Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y (2020) Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa478
Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, Liu X, Wei L, Truelove SA, Zhang T, Gao W, Cheng C, Tang X, Wu X, Wu Y, Sun B, Huang S, Sun Y, Zhang J, Ma T, Lessler J, Feng T (2020) Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. https://doi.org/10.1016/s1473-3099(20)30287-5
Cai J, Xu J, Lin D, Yang Z, Xu L, Qu Z, Zhang Y, Zhang H, Jia R, Liu P, Wang X, Ge Y, Xia A, Tian H, Chang H, Wang C, Li J, Wang J, Zeng M (2020) A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa198
CDC COVID-19 Response Team (2020) Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. https://doi.org/10.15585/mmwr.mm6914e4
Centers for Disease Control and Prevention (2020) Evaluating and testing persons for coronavirus disease 2019 (COVID-19). Centers for Disease Control and Prevention. Accessed 25 March 2020
Choi SH, Kim HW, Kang JM, Kim DH, Cho EY (2020) Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr 63:125–132
Ge Y, Cai J, Wang X, Yao W, Shen J, Zhu Q, Wang X, Zeng M (2012) Childhood influenza in the outpatient setting in Shanghai, China. Pediatr Infect Dis J 31:e111–e116
Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, Ma H, Chen W, Lin Y, Zheng Y, Wang J, Hu Z, Yi Y, Shen H (2020) Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 63:706–711
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207
Loeb M, Singh PK, Fox J, Russell ML, Pabbaraju K, Zarra D, Wong S, Neupane B, Singh P, Webby R, Fonseca K (2012) Longitudinal study of influenza molecular viral shedding in Hutterite communities. J Infect Dis 206:1078–1084
Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK, Chinese Pediatric Novel Coronavirus Study T (2020) SARS-CoV-2 infection in children. N Engl J Med 382:1663–1665
National Health Commission of The People’s Republic of China (2019) Interim protocol of diagnosis and treatment of 2019 novel coronavirus-associated pneumonia (the seventh version). National Health Commission of The People’s Republic of China, Beijing, China
National Health Commission of The People’s Republic of China (2020) Coronavirus disease 2019 (COVID-19) situation report in China: data as reported by 24:00 11 March 2020. National Health Commission of The People’s Republic of China, Beijing
National Health Commission and State Administration of Chinese Traditional Medicine (2020) Clinical protocols for the diagnosis and treatment of COVID-19 V7. Beijing, China
Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D (2020) Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 20:689–696
Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wolfel R, Hoelscher M (2020) Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 382:970–971
Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R (2020) High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 26:1470–1477
Shanghai Municipal Health Commission (2020) Coronavirus disease 2019 (COVID-19) situation report in Shanghai: data as reported by 24:00 30 April 2020. Shanghai Municipal Health Commission, Shanghai
Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, Moraleda C, Calvo C (2020) Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2020.1346
Tang YW, Schmitz JE, Persing DH, Stratton CW (2020) Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 58:e00512-20
Wang X, Cai J, Yao W, Zhu Q, Zeng M (2015) Socio-economic impact of influenza in children: a single-centered hospital study in Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi 36:27–30
Wang D, Ju XL, Xie F, Lu Y, Li FY, Huang HH, Fang XL, Li YJ, Wang JY, Yi B, Yue JX, Wang J, Wang LX, Li B, Wang Y, Qiu BP, Zhou ZY, Li KL, Sun JH, Liu XG, Li GD, Wang YJ, Cao AH, Chen YN (2020) Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi 58:269–274
World Health Organization (WHO) (2020a) Coronavirus disease 2019 (COVID-19) situation report—51. Data as reported by national authorities by 10 AM CET 11 March 2020. World Health Organization (WHO), Geneva
World Health Organization (WHO) (2020b) Molecular assays to diagnose COVID-19: summary table of available protocols. WHO. https://www.who.int/who-documents-detail/molecular-assays-to-diagnose-covid-19-summary-table-of-available-protocols. Accessed 06-08 2020
World Health Organization (WHO) (2020c) Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). World Health Organization (WHO), Geneva, Switzerland
Yong Z, Cao C, Shuangli Z, Chang S, Dongyan W, Jingdong S, Yang S, Wei Z, Zijian F, Guizhen W, Jun X, Wenbo X (2020) Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19). China CDC Wkly 2:123–124
Zhang J, Litvinova M, Liang Y, Wang Y, Wang W, Zhao S, Wu Q, Merler S, Viboud C, Vespignani A, Ajelli M, Yu H (2020) Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. https://doi.org/10.1126/science.abb8001
Acknowledgements
This work was supported by a grant from Science and Technology Commission of Shanghai Municipality (Grant number: 20JC1410203) and a grant from Shanghai Municipal Health Commission (Grant Number: 202040099).
Author information
Authors and Affiliations
Contributions
MZ and XZ conceptualized and designed the study, and finalized the manuscript; JC, XW, JZ and YG performed the experiments; JX, HT, HC, AX, JW, JZ, ZW, JL, CW collected data and carried out the initial analyses; JC and MZ drafted the manuscript; JW, QZ contributed to design the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors. Informed consent from parents was not required by the ethics committee because all data were de-identified and not involved in personal privacy.
Rights and permissions
About this article
Cite this article
Cai, J., Wang, X., Zhao, J. et al. Comparison of Clinical and Epidemiological Characteristics of Asymptomatic and Symptomatic SARS-CoV-2 Infection in Children. Virol. Sin. 35, 803–810 (2020). https://doi.org/10.1007/s12250-020-00312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-020-00312-4