Abstract
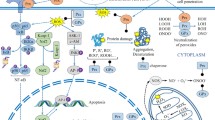
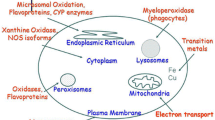
Free radical mediated pathologies occupy a special place in medical semiology and in mechanistic interpretation of diseases. Free radicals, or better reactive oxygen species (ROS) or reactive nitrogen species (RNS) play also an important role in cell signaling. This is the basis of the ambivalent (Jekyll – Hyde) situation of ROS in biology and pathology. Aging itself is attributed by a popular theory to free radicals. A number of ROS – scavenging substances and procedures were described without however reaching credibility for their therapeutic value. An interesting exception is the xanthine oxido - reductase produced ROS and their role in cardiovascular disease. Allopurinol inhibition of xanthine oxido – reductase was shown to be efficient in some cases of cardiovascular diseases. Another important aspect of xanthine oxido – reductase produced ROS is their antibacterial capacity considered to be of importance with newborns fed on milk rich in this enzyme as well as at the gastrointestinal barrier. This ambivalent role of xanthine oxido – reductase justifies this review on the basic enzymatic mechanisms involved, derived ROS production, their role in the above mentioned biological processes and especially the interest of the inhibition of this enzyme as a preventive or curative measure in some cardiovascular pathologies.




Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- XOR:
-
Xanthine oxido reductase
- XO:
-
Xanthine oxidase
- XD:
-
Xanthine dehydrogenase
- AO:
-
Aldehyde oxidase
- MFE:
-
Molybdo flavo enzymes
- UA:
-
Uric acid
- NOS:
-
Nitric oxide synthetase
- CVD:
-
Cardio vascular disease
- IR:
-
Ischemia reperfusion
- MI:
-
Myocardial infarct
- HF:
-
Heart failure
- BP:
-
Blood pressure
- EPR:
-
Electron paramagnetic resonance
- NAD:
-
Nicotine adenine dinucleotide
- Kb:
-
1000 base-unit in DNA
- SOD:
-
Superoxide dismutase
References
Robert L, Labat-Robert J, Robert AM (2010) Vieillissement cellulaire, télomères et maladies liées à l’âge. Médecine & Longévité 2:151–161. doi:10.1016/j.mlong.2010.07.005
Harman D (1955) Aging — a theory based on free radical and information theory U.C.R.L. Publ., 3078, Univ. of Calif.
Comfort A (1979) The biology of senescence, 3rd edn. Churchill Livingstone, Edinburgh & London, 1979
Emerit I, Eds BC (1992) Free radicals and aging. Birkhäuser Verlag, Basel, 1992
Edeas M (2009) Anti-oxydants, controverses et perspectives: comment expliquer l’échec des etudes cliniques utilisant des anti-oxydants. J Soc Biol 203:271–280
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Martin HM, Hancock JT, Salisbury V, Harrison R (2004) Mini-review. Role of xanthine oxydoreductase as an antimicrobial agent. Infect Immun 72:4933–4939
Miller SL, Urey HC (1959) Organic compound synthesis on the primitive earth. Science 130:245–251
Garattini E, Mendel R, Romao MJ, Wright R, Terao M (2003) Review article. Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem J 372:15–32
Drury AN, Szent-Györgyi A (1929) The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol 68:213–237
Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during inflammation. New Engl J Med 367:2322–2333
Patton S, Keenan TW (1975) The milk fat globule membrane. Biochim Biophys Acta 415:273–309
Dowben RM, Brunner JR, Philpott DE (1967) Studies on milk fat globule membranes. Biochim Biophys Acta 135:1–10
Polonovski M, Robert M, Robert L (1950) Cinétique de la révélation de la xanthinedéhydrase du lait de vache. Bull Soc Chim Biol 32:862–867
Polonovski M, Baudu L, Robert M, Robert L (1950) Données nouvelles sur la révélation de la xanthine-déhydrase du lait par les agents chimiques. Bull Soc Chim Biol 32:855–861
Polonovski M, Robert L, Robert M (1950) Une nouvelle méthode d’extraction et de purification de la xanthine déhydrase du lait. Bull Soc Chim Biol 32:868–871
Robert L, Basset J (1953) Action des hautes pressions sur la xanthinedéhydrase du lait. Bull Soc Chim Biol 35:1375–1380
Robert L, Nolla N (1953) Action des ultrasons sur la xanthinedéhydrase du lait. Bull Soc Chim Biol 35:1363–1373
Robert L, Polonovski M (1955) Activation and inactivation of milk xanthineoxydase by physicochemical means. In: Discussions of the Faraday Society, N° 20, The Physical Chemistry of Enzymes The Aberdeen University Press Ltd; 6 Upper Kirkgate, Aberdeen, pp 54–65
Avis PG, Berger F, Bray RC, Shooter KV (1954) A crystalline material with xanthine oxidase activity. Nature 173:1230–1233
Avis PG, Bergel F, Bray RC (1955) Cellular constituents. The chemistry of xanthine oxidase. Pat I. The preparation of a crystalline xanthine oxidase from cow’s milk. J Chem Soc April 1100–1105
Avis PG, Bergel F, Bray RC, James DWF, Shooter KV (1956) Cellular constituents. The chemistry of xanthine oxidase. Part II. The homogeneity of crystalline metalloflavoprotein fractions. J Chem Soc 252:1212–1219
Avis PG, Bergel F, Bray RC (1956) Cellular constituents. The chemistry of xanthine oxidase. Part III. Estimations of the co-factors and the catalytic activities of enzyme fractions from cow’s milk. J Chem Soc 253:1219–1226
Bergel F, Bray RC (1959) The chemistry of xanthine oxidase. 4. The problems of enzyme inactivation and stabilisation. Biochem J 73:182–192
Bray RC, Chisholm AJ, Hart LI, Meriwether LS, Watts DC (1966) Studies on the composition and mechanism of action of milk xanthine oxidase. In: Slater EC (ed) Flavins and flavoproteins. Elsevier Publishing Company, Amsterdam, pp 117–132
Haddow A, de Lamirande G, Bergel F, Bray RC, Gilbert DA (1958) Anti-tumour and biochemical effects of purified bovine xanthine oxidase in C3H and C mice. Nature 182:1144–1146
Mackler B, Mahler HR, Green DE (1954) Studies on metalloflavoproteins. I. Xanthine oxydase. A molybdoflavoprotein. J Biol Chem 210:149–164
Berry CE, Hare JM (2004) Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555:589–606
Granger DN, Rutili G, McCord J (1981) Superoxide radicals in feline intestinal ischemia. Gastroenterology 81:22–29
Carden DI, Granger DN (2000) Pathology of ischemia-reperfusion injury. J Pathol 190:255–266
Harrison R (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33:774–797
Granger DN, Rutili G, McCord JM (1981) Superoxide radicals in feline intestinal ischemia. Gastroenterology 81:22–29
Abadeh S, Killacky J, Benboubetra M, Harrison R (1992) Purification and partial characterization of xanthine oxidase from human milk. Biochim Biophys Acta 1117:25–32
Vorbach C, Scriven A, Capecchi MR (2002) The housekeeper gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev 16:3223–3235
Simmonds HA (1994) Purine and pyrimidine disorders. In: Holton JS (ed) The Inherited metabolic diseases, 2nd edn. Churchill Livingstone, New York, pp 297–307
Delbarre F, Cartier P, Auscher C, De Géry A, Hamet M (1970) Gouttes enzymopathiques. Dyspurinies par déficit en Hypoxanthine-Guanine-Phosphoribosyl-Transférase. Fréquence et caractères cliniques de l’anenzymose. Presse Med 78:729–734
Ea H-K, Bardin T, Jinnah HA, Aral B, Lioté F, Ceballos - Picot I (2009) Severe Gouty Arthritis and mild neurologic symptoms due to F199C, a newly identified variant of the hypoxanthine guanine phosphoribosyltransferase. Arthritis Rheum 60:2201–2220
Lewers JC, Ceballos-Picot I, Shirley TL, Mockel L, Egami K, Jinnah HA (2008) Consequences of impaired purine recycling in dopaminergic neurons. Neuroscience 152:761–772
Bollée G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, Deteix P, Daudon M, Knebelmann B, Ceballos-Picot I (2010) Phenotype and genotype characterization of adenine phosphoribosyltransfetrase deficiency. J Am Soc Nephrol 4:679–688
Jinnah HA, Ceballos-Picot I, Torres RJ, Visser JE, Schretlen DJ, Verdu A, Larovere LE, Chen S-J, Cossu A et al (2010) Attenuated variants of Lesch-Nyhan disease. Brain 133:671–689
Li H, Samouilov A, Liu X, Zweier JL (2003) Characterization of the magnitude and kinetics of xanthine oxidase – catalysed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 42:1150–1159
Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM (2001) Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem 276:14359–14365
Adachi T, Fukushima T, Usami Y, Hirano K (1993) Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial cell surface. Biochem J 289:523–527
Brown JM, Terrada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE (1988) Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Invest 81:1297–1301
Wang W, Sawicki G, Schulz R (2002) Peroxinitrite – induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res 53:165–174
Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JRB, Sawicki G, Schulz R (2002) Intracellular action of matrix metalloproteinase - 2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 106:1543–1549
Campbell DL, Stamler JS, Strauss HC (1996) Redox modulation of L-type calcium channels in ferret ventricular myocytes dual mechanism regulation by nitric oxide and S-nitrosothiols. J General Physiol 108:277–293
Ohara Y, Peterson TE, Harrison DG (1993) Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91:2546–2551
Burns CM, Wortmann RL (2012) Latest evidence on gout management: what the clinician needs to know. Ther Adv Chronic Dis 3(6):271–286
Truglio JJ, Theis K, Leimkühler S, Rappa R, Rajagopalan KV, Kisker C (2002) Crystal structures of the active and alloxanthine-inhibited forms of xanthine dehydrogenase from Rhodobacter capsulatus. Structure 10:115–125
Sanders S, Eisenthal RS, Harrison R (1997) NADH oxidase activity of human xanthine oxidoreductase –generation of superoxide anion. Eur J Biochem 245:541–548
Brown JM, Terada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE (1988) Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated perfused rat hearts. J Clin Invest 81:1297–1301
Grisham MB, Hernandez LA, Granger DN (1986) Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol 251:G567–G574
Gimpel JA, Lahpor JR, van der Molen A, Damen J, Hitchcock JF (1995) Reduction of reperfusion injury of human myocardium by allopurinol: a clinical study. Free Radic Biol Med 19:251–255
Guan W, Osanai T, Kamada T, Hanada H, Ishizaka H, Onodera H, Iwasa A, Fujita N, Kudo S, OhkuboT OK (2003) Effect of allopurinol pretreatment on free radical generation after primary coronary angioplasty for acute myocardial infarction. J Cardiovasc Pharmacol 41:699–705
Butler R, Morris AD, Belch JJF, Hill A, Struthers AD (2000) Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35:746–751
Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Iwasaki T (2003) Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA 88:10045–10048
Kögler H, Fraser H, McCune S, Altschuld R, Marban E (2003) Disproportionate enhancement of myocardial contractility by the xanthine oxidase inhibitor oxypurinol in failing rat myocardium. Cardiovasc Res 59:582–592
Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM (2001) Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 104:2407–2411
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–18 21
Galiardi AC, Miname MH, Santos RD (2009) Uric acid: a marker of increased cardiovascular risk. Atherosclerosis 202:11–17
Krishnan E, Baker JF, Furst DE, Schumacher HR (2006) Gout and the risk of acute myocardial infarction. Arthritis Rheum 54:2688–2696
Krishnan E, Pandya BJ, Lingala B, Hariri A, Dabbous O (2012) Hyperuricemia and untreated gout are poor prognostic markers among those with a recent acute myocardial infarction. Arthritis Res Ther 14:R10
Baker JF, Schumacher HR, Krishnan E (2007) Serum uric acid level and risk for peripheral arterial disease: analysis of data from the multiple risk factor intervention trial. Angiology 58:450–457
Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller RH (2008) Long-term cardiovascular mortality among middle – aged men with gout. Arch Intern Med 168:1104–1110
Choi HK, Curhan G (2007) Independent impact of gout on mortality and risk for coronary heart disease. Circulation 116:894–900
Hanassoulis G, Brophy JM, Richard H, Pilote L (2010) Gout, allopurinol use and heart failure outcomes. Arch Intern Med 170:1358–1364
Noman A, Ang DS, Ogston S, Lang CC, Struthers AD (2010) Effect of high - dose allopurinol on exercise in patients with chronic stable angina: a randomized, placebo controlled crossover trial. Lancet 375:2161–2167
Feig DI, Soletsky B, Johnson RJ (2008) Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 300:924–932
Kelkar A, Kuo A, Frishman WH (2011) Allopurinol as a cardiovascular drug. Cardiol Rev 19:265–271
Acknowledgments
The project of this review was suggested by our colleague Dr Annick Alperovitch, Emeritus Research Director at INSERM, eminent specialist of cardiovascular epidemiology. She also helped us with the content of section VII on the epidemiology of CVD as related to XO-
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robert, A.M., Robert, L. Xanthine Oxido-Reductase, Free Radicals and Cardiovascular Disease. A Critical Review. Pathol. Oncol. Res. 20, 1–10 (2014). https://doi.org/10.1007/s12253-013-9698-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-013-9698-x