Abstract
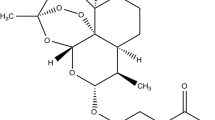
Dihydroartemisinin (DHA) is a major metabolite of artemisinin and its derivatives, including arteether, artemether, and artesunate. To improve the solubility and stability of poorly soluble DHA, we prepared inclusion complexes with hydroxypropyl-β-cyclodextrin (HPβCD) and recrystalized DHA to study its thermal stability. The complexes were characterized by differential scanning calorimetery (DSC), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction patterns (XRD), thermal stability, phase, and equilibrium solubility studies. Pure DHA was crystalline and remained crystalline after recrystallization, but its unit cell dimensions changed as exhibited by XRD. DHA-HPβCD complexes showed a phase transitions towards amorphous in DSC thermograms, FTIR spectra, and XRD patterns. The phase solubility profiles of complexes prepared in water, acetate buffer, and phosphate buffers were classified as AL-type, indicating the formation of a 1:1 stoichiometric inclusion complex. The equilibrium solubility of DHA was enhanced as a function of HPβCD concentration. DHA-HPβCD complexes showed an 89-fold increase in solubility compared to DHA. Solubilities of complexes containing 275.1 mM HPβCD in water, acetate buffer (pH 3.0), and phosphate buffer (pH 3.0 and 7.4) were 10.04, 7.96, 6.30, and 11.61 mg/ml, respectively. Hydrogen bonding was found between DHA and HPβCD, and it was stronger in complexes prepared in water than in buffers. However, the ÄH values were higher in buffer than water. DHA-HPβCD complexes prepared using commercial (untreated) or recrystallized DHA (no detectable impurity) showed a 40% increase in thermal stability (50°C) and a 29-fold decrease in hydrolysis rates compared with DHA. The rank order of stability constants (Ks) was: water, acetate buffer (pH 3.0), phosphate buffer (pH 3.0), and phosphate buffer (pH 7.4). Thus, HPβCD complexation with recrystalized DHA increases DHA solubility and stability.
Similar content being viewed by others
References
Al Omari, M. M., Zughul, M. B., Davies, J. E. D., and Badwan, A. A., effect of buffer species on the inclusion complexation of acidic drug celecoxib with cyclodextrin in solution. J. Inclu. Phen. Macrocy. Chem., 55, 247–254 (2006).
Ansari, M. T. and Sunderland, V. B., Solid dispersions of dihydroartemisinin in polyvinylpyrrolidone. Arch. of Pharm. Res., 31(3), 390–398 (2008).
Baboota, S., Khanna, R., Agarwal, S. P., Ali, J., and Ahuja, A., Cyclodextrins in drug delivery systems: An update. PharmaInfo. net. (2003).
Backensfeld, T., Muller, B. W., and Kolter, K., Interaction of NSA with cyclodextrins and Hydroxypropyl cyclodextrin derivatives. Int. J. Pharm., 74, 85–93 (1991).
Cabral Marques, H., Hadgraft, J., and Kllaway, I., Studies of cyclodextrin inclusion complexes. 1. The salbutamol-cylodex trin complex as studied by phase solubility and DSC. Int. J. Pharm., 63, 259–266 (1990).
Chen, X., Chen, R., Guo, Z., Li, C., and Li, P., The preparation and stability of the inclusion complex of astaxanthin with β-cyclodextrin. Food, Chem., 101, 1580–1584 (2007)
Cirri, M., Maestrelli, F., Corti, G., Furlanetto, S., and Mura, P., Simultaneous effect of cyclodextrins complexation, pH and hydrophilic polymers on naproxen solubilization. J. Pharm. BioMed Anal., 42, 126–131 (2006).
de Araujo, D. R., Tsuneda, S. S., Cereda, C. M. S., Carvalho, F. D. G. F., Prete, P. S. C., Fernandes, S. A., Yokaichiya, F., Franco, M. K. K. D., Mazzaro, I., Fraceto, L. F., Braga, A. F. A., and Paula, E., Development of pharmacological evaluation of ropivacaine-2-hydroxypropyl-β-cyclodextrin inclusion complexes. Eur. J. Pharm. Sci., 33, 60–71 (2008).
de Vries, P. J. and Dien, T. K., Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs, 52(6), 818–836 (1996).
Dordunoo, S. K. and Burt, H. M., Solubility and stability of taxol: effects of buffers and cyclodextrins. Int. J. Pharm., 133, 191–201 (1996).
Elisabeth, E., The history of qing hao in the Chinese materia medica.Trans. Roy. Soc. Trop. Med. Hyg., 100, 505–508 (2006).
Fernandes, C. M., Vieira, M. T., and Veiga, F. J., Physicochemical characterization and in vitro dissolution behavior of nicardipine-cyclodextrins inclusion compounds. Eur. J. Pharm. Sci., 15, 79–88 (2002).
Higuchi, T. and Connors, K. A., Phase solubility technique. Adv. Anal. Chem. Instrum., 4, 117–212 (1965).
Illapakurthy, A. C., Sabnis, Y. A., Avery, B. A., Avery, M. A., and Wyandt, C. M., Interaction of artemisinin and its related compounds with Hydroxypropyl-β-cyclodextrin in solution state: Experimental and Molecular-Modeling studies. J. Pharm. Sci., 92, 649–655 (2003).
Kang, J., Kumar, V., Yang, D., Chowdhary, P. R., and Hohl, R. J., 2002. Cyclodextrin complexation: influence on the solubility, and cytotoxicity of camptothecin, an antineoplastic agent. Eur. J. Pharm. Sci., 15, 163–170 (2002).
Li, Q. C., Peggins, J. O., Fleckenstein, L. L., Masonic, K., Heiffer, M. H., and Brewer, T. G., The pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J. Pharm. Pharmacol., 50, 173–182 (1998).
Liu, X., Lin, H., Thenmozhiyal, I. C., Chan, S. Y., and Ho, P. C., Inclusion of Acitretin into cyclodextrins: Phase solubility, photostability, and physicochemical characterization. J. Pharm. Sci., 92(12), 2449–2457 (2003).
Loftsson, T. and Duchene, D., Cyclodextrins and their pharmaceutical applications. Int. J. Pharm., 329, 1–11 (2007).
Loftsson, T. and Brewster, M., Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci., 85(10), 1017–1025 (1996).
Miyake, K., Irie, T., Arima, H., Hirayama, F., Uekama, K., Hirano, M., and Okamoto, Y., Characterization of itroconazole/2-hydroxypropyl-β-cyclodextrin inclusion complex in aqueous propylene glycol solution. Int. J. Pharm., 179(2), 237–245 (1999).
Mochida, K., Kagita, A., Matsui, Y., and Date, Y., Effects of Inorganic Salts on the Dissociation of a Complex of β-Cyclodextrin with an Azo Dye in an Aqueous Solution. Bull. Chem. Soc. Japn., 46, 3703–3707 (1973).
Monographs for antimalarial drugs. In. International Pharmacopoeia, 215 (2004).
Nagarsenker, M. S. and Joshi, M. S., Celecoxib-cyclodextrin systems: Characterization and evaluation of In vitro and In vivo advantage. Drug Dev. Ind. Pharm., 31, 169–178 (2005).
Passmore, P. and Sunderland, V. B., Quality assurance in formulation development and analytical methods for combination antimalarial products. A report of School of Pharmacy, Curtin University of Technology, Australia (2003).
Peeters, J., Neeskens, P., Tollenaere, J. P., Remoortere, P. V., and Brewster, M. E., Characterization of the interaction of 2-hydroxypropyl-β-cyclodextrin with iteraconazole at pH 2,4, and 7. J. Pharm. Sci., 91(6), 1414–1422 (2002).
Perlovich, G. L., Skar, M., and Bauer-Brandl, A., Driving forces and the influence of the buffer composition on the complexation reaction between ibuprofen and HPβCD. Eur. J. Pharm. Sci., 20, 197–200 (2003).
Pogany, D., “Stability. In. Prequalification of antimalarial drug products. Geneva. 21–22 (2004).
Rajewski, R. A., Traiger, G., Bresnahan, J., Jaberaboansari, P., Stella, V. J., and Thompson, D. O., Preliminary safety evaluation of parenterally administered sulfoalkyl ether β-cyclodextrin derivatives. J. Pharm. Sci., 84, 927–932 (1995).
Rawat, S. and Jain, S. K., Solubility enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur. J. Pharm. Biopharm., 57, 263–267 (2004).
Reddy, M. N., Rehana, T., Ramakrishna, S., Diwan, P. V., and Chowdary, K. P. R., Beta-cyclodextrin complexes of celecoxib: molecular-modeling characterization and dissolution studies. AAPS Pharm. Sci., 6, 7–13 (2004).
Sethia, S. and Squillante, E., Solid dispersion of carbamazepine in PVP by conventional solvent evaporation and supercritical methods. Int. J. Pharm., 272, 1–10 (2004).
Stella, V. J. and Rajewski, R. A., Cyclodextrins: their future in drug formulation and delivery. Pharm. Res., 14, 556–567 (1997).
Tommasini, S., Calabro, M. L., Raneri, D., Ficarra, P., and Ficarra, R., Solubilization of non-steroidal anti-inflammatory. J. Pharm. Biomed. Anal., 36, 327–333 (2004).
Usuda, M., Endo, T., Nagase, H., Tomono, K., and Ueda, H., Interaction of antimalarial agent artemisinin with cyclodextrins. Drug Dev. Ind. Pharm., 26(6), 613–619 (2000).
Wojcik, J. F. and Rohrbach, R. P., Small anion binding to cycloamylose. Equilibrium constants. J. Phys. Chem., 79(21),2251–2253 (1975).
Yi, Z., Zhao, C., Huang, Z., Chen, H., and Yu, J., investigation of buffer cyclodextrin systems. Phys. Chem. Chem. Phys., 1, 441–448 (1999).
Zingone, G. and Rubessa, F., Preformulation study of the inclusion complex warfarin-β-cyclodextrin. Int. J. Pharm., 291, 3–10 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ansari, M.T., Iqbal, I. & Sunderland, V.B. Dihydroartemisinin-cyclodextrin complexation: Solubility and stability. Arch. Pharm. Res. 32, 155–165 (2009). https://doi.org/10.1007/s12272-009-1130-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-009-1130-4