Abstract
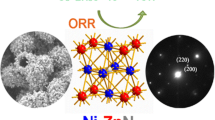
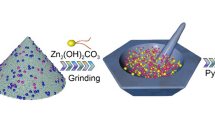
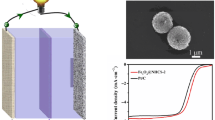
Design and fabrication of cost-effective transition metal and their oxides-based nanocomposites are of paramount significance for metal-air batteries and water-splitting. However, the traditional optimized designs for nanostructure are complicated, low-efficient and underperform for wide-scale applications. Herein, a novel hierarchical framework of hollow Ni/NiFe2O4-CNTs composite microsphere forcibly-assembled by zero-dimensional (0D) Ni/NiFe2O4 nanoparticle (< 16 nm) and one-dimensional (1D) self-supporting CNTs was fabricated successfully. Benefitted from the unique nanostructure, such monohybrids can achieve remarkable oxygen evolution reaction (OER) performance in alkaline media with a low overpotential and superior durability, which exceeds most of the commercial catalysts based on IrO2/RuO2 or other non-noble metal nanomaterials. The enhanced OER performance of Ni/NiFe2O4-CNTs composite is mainly ascribed to the increased catalytic activity and the optimized conductivity induced by the effects of strong hierarchical coupling and charge transfers between CNTs and Ni/NiFe2O4 nanoparticles. These effects are greatly boosted by the polarized heterojunction interfaces confirmed by electron holography. The density functional theory (DFT) calculation indicates the epitaxial Ni further enriches the intrinsic electrons contents of NiFe2O4 and thus accelerates absorption/desorption kinetics of OER intermediates. This work hereby paves a facile route to construct the hollow composite microsphere with excellent OER electrocatalytic activity based on non-noble metal oxide/CNTs.

Similar content being viewed by others
References
Deng, S. J.; Zhong, Y.; Zeng, Y. X.; Wang, Y. D.; Wang, X. L.; Lu, X. H.; Xia, X. H.; Tu, J. P. Hollow TiO2@Co9S8 core-branch arrays as bifunctional electrocatalysts for efficient oxygen/hydrogen production. Adv. Sci. (Weinh)2018, 5, 1700772.
Cheng, G. H.; Kou, T. T.; Zhang, J.; Si, C. H.; Gao, H.; Zhang, Z. H. O22−/O− functionalized oxygen-deficient Co3O4 nanorods as high performance supercapacitor electrodes and electrocatalysts towards water splitting. Nano Energy2017, 38, 155–166.
Tang, C.; Wang, B.; Wang, H. F.; Zhang, Q. Defect engineering toward atomic Co-Nx-C in hierarchical graphene for rechargeable flexible solid Zn-air batteries. Adv. Mater.2017, 29, 1703185.
Si, C. H.; Zhang, Y. L.; Zhang, C. Q.; Gao, H.; Ma, W. S.; Lv, L. F.; Zhang, Z. H. Mesoporous nanostructured spinel-type MFe2O4 (M = Co, Mn, Ni) oxides as efficient bi-functional electrocatalysts towards oxygen reduction and oxygen evolution. Electrochim. Acta2017, 245, 829–838.
Li, P. S.; Duan, X. X.; Kuang, Y.; Li, Y. P.; Zhang, G. X.; Liu, W.; Sun, X. M. Tuning electronic structure of NiFe layered double hydroxides with vanadium doping toward high efficient electrocatalytic water oxidation. Adv. Energy Mater.2018, 8, 1703341.
Wang, Z.; Ang, J. M.; Zhang, B. W.; Zhang, Y. F.; Ma, X. Y. D.; Yan, T.; Liu, J.; Che, B. Y.; Huang, Y. Z.; Lu, X. H. FeCo/FeCoNi/N-doped carbon nanotubes grafted polyhedron-derived hybrid fibers as bifunctional oxygen electrocatalysts for durable rechargeable zinc-air battery. Appl. Catal. B: Environ.2019, 254, 26–36.
Jiang, H.; Gu, J. X.; Zheng, X. S.; Liu, M.; Qiu, X. Q.; Wang, L. B.; Li, W. Z.; Chen, Z. F.; Ji, X. B.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci.2019, 72, 322–333.
Finke, C. E.; Omelchenko, S. T.; Jasper, J. T.; Lichterman, M. F.; Read, C. G.; Lewis, N. S.; Hoffmann, M. R. Enhancing the activity of oxygen-evolution and chlorine-evolution electrocatalysts by atomic layer deposition of TiO2. Energy Environ. Sci, 2019, 72, 358–365.
Zhen, D. X.; Zhao, B. T.; Shin, H. C.; Bu, Y. F.; Ding, Y.; He, G. H.; Liu, M. L. Electrospun porous perovskite La0.6Sr0.4Co1−xFexO3−δ nanofibers for efficient oxygen evolution reaction. Adv. Mater. Interfaces2017, 4, 1700146.
Niu, S. Q.; Sun, Y. C.; Sun, G. J.; Rakov, D.; Li, Y. Z.; Ma, Y.; Chu, J. Y.; Xu, P. Stepwise electrochemical construction of FeOOH/Ni(OH)2 on Ni foam for enhanced electrocatalytic oxygen evolution. ACS Appl. Energy Mater.2019, 2, 3927–3935.
Jin, H. Y.; Wang, J.; Su, D. F.; Wei, Z. Z.; Pang, Z. F.; Wang, Y. In situ cobalt-cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc.2015, 137, 2688–2694.
Mahala, C.; Sharma, M. D.; Basu, M. 2D nanostructures of CoFe2O4 and NiFe2O4: Efficient oxygen evolution catalyst. Electrochim. Acta2018, 273, 462–473.
Li, T. F.; Lv, Y. J.; Su, J. H.; Wang, Y.; Yang, Q.; Zhang, Y. W.; Zhou, J. C.; Xu, L.; Sun, D. M.; Tang, Y. W. Anchoring CoFe2O4 nanoparticles on N-doped carbon nanofibers for high-performance oxygen evolution reaction. Adv. Sci. (Weinh)2017, 4, 1700226.
Yu, L.; Yang, J. F.; Guan, B. Y.; Lu, Y.; Lou, X. W. Hierarchical hollow nanoprisms based on ultrathin Ni-Fe Layered double hydroxide nanosheets with enhanced electrocatalytic activity towards oxygen evolution. Angew. Chem., Int. Ed.2018, 57, 172–176.
Zhou, D. J.; Cai, Z.; Lei, X. D.; Tian, W. L.; Bi, Y. M.; Jia, Y.; Han, N. N.; Gao, T. F.; Zhang, Q.; Kuang, Y. et al. NiCoFe-layered double hydroxides/N-doped graphene oxide array colloid composite as an efficient bifunctional catalyst for oxygen electrocatalytic reactions. Adv. Energy Mater.2018, 8, 1701905.
Zhao, S. L.; Li, M.; Han, M.; Xu, D. D.; Yang, J.; Lin, Y.; Shi, N. E.; Lu, Y. N.; Yang, R.; Liu, B. T. et al. Defect-rich Ni3FeN nanocrystals anchored on N-doped graphene for enhanced electrocatalytic oxygen evolution. Adv. Funct. Mater.2018, 28, 1706018
Fu, G. T.; Cui, Z. M.; Chen, Y. F.; Li, Y. T.; Tang, Y. W.; Goodenough, J. B. Ni3Fe-N doped carbon sheets as a bifunctional electrocatalyst for air cathodes. Adv. Energy Mater.2017, 7, 1601172.
Wang, W. H.; Yang, Y.; Huan, D. M.; Wang, L. K.; Shi, N.; Xie, Y.; Xia, C. R.; Peng, R. R.; Lu, Y. L. An excellent oer electrocatalyst of cubic SrCoO3−δ prepared by a simple F-doping strategy. J. Mater. Chem. A2019, 7, 12538–12546.
Yue, X.; Jin, Y. S.; Shen, P. K. Highly stable and efficient non-precious metal electrocatalysts of tantalum dioxyfluoride used for the oxygen evolution reaction. J. Mater. Chem. A2017, 5, 8287–8291.
Li, M.; Lu, M. J.; Yang, J. R.; Xiao, J.; Han, L. N.; Zhang, Y. J.; Bo, X. J. Facile design of ultrafine CuFe2O4 nanocrystallines coupled porous carbon nanowires: Highly effective electrocatalysts for hydrogen peroxide reduction and the oxygen evolution reaction. J. Alloys Compd.2019, 809, 151766.
Han, M. N.; Shi, M. J.; Wang, J.; Zhang, M. L.; Yan, C.; Jiang, J. T.; Guo, S. H.; Sun, Z. Y.; Guo, Z. H. Efficient bifunctional Co/N dual-doped carbon electrocatalysts for oxygen reduction and evolution reaction. Carbon2019, 153, 575–584.
Wang, X.; Huang, X. K.; Gao, W. B.; Tang, Y.; Jiang, P. B.; Lan, K.; Yang, R. Z.; Wang, B.; Li, R. Metal-organic framework derived CoTe2 encapsulated in nitrogen-doped carbon nanotube frameworks: A high-efficiency bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A2018, 6, 3684–3691.
Suryawanshi, U. P.; Suryawanshi, M. P.; Ghorpade, U. V.; Shin, S. W.; Kim, J.; Kim, J. H. An earth-abundant, amorphous cobalt-iron-borate (Co-Fe-Bi) prepared on Ni foam as highly efficient and durable electrocatalysts for oxygen evolution. Appl. Surf. Sci.2019, 495, 143462.
Liu, G.; Wang, K. F.; Gao, X. S.; He, D. Y.; Li, J. P. Fabrication of mesoporous NiFe2O4 nanorods as efficient oxygen evolution catalyst for water splitting. Electrochim. Acta2016, 211, 871–878.
He, K.; Tadesse Tsega, T.; Liu, X.; Zai, J. T.; Li, X. H.; Liu, X. J.; Li, W. H.; Ali, N.; Qian, X. F. Utilizing the space-charge region of the FeNi-LDH/CoP p-n junction to promote performance in oxygen evolution electrocatalysis. Angew. Chem., Int. Ed.2019, 58, 11903–11909.
Panda, C.; Menezes, P. W.; Yao, S. L.; Schmidt, J.; Walter, C.; Hausmann, J. N.; Driess, M. Boosting electrocatalytic hydrogen evolution activity with a NiPt3@NiS heteronanostructure evolved from a molecular nickel-platinum precursor. J. Am. Chem. Soc.2019, 141, 13306–13310.
Xiong, Y.; Xu, L. L.; Jin, C. D.; Sun, Q. F. Interface-engineered atomically thin Ni3S2/MnO2 heterogeneous nanoarrays for efficient overall water splitting in alkaline media. Appl. Catal. B: Environ.2019, 254, 329–338.
Guo, H. L.; Feng, Q. C.; Zhu, J. X.; Xu, J. S.; Li, Q. Q.; Liu, S. L.; Xu, K. W.; Zhang, C.; Liu, T. X. Cobalt nanoparticle-embedded nitrogen-doped carbon/carbon nanotube frameworks derived from a metal-organic framework for tri-functional ORR, OER and HER electrocatalysis. J. Mater. Chem. A2019, 7, 3664–3672.
Dong, C. Q.; Kou, T. Y.; Gao, H.; Peng, Z. Q.; Zhang, Z. H. Eutectic-derived mesoporous Ni-Fe-O nanowire network catalyzing oxygen evolution and overall water splitting. Adv. Energy Mater.2018, 8, 1701347.
Shan, J. Q.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S. Z. Transition-metal-doped RuIr bifunctional nanocrystals for overall water splitting in acidic environments. Adv. Mater.2019, 31, 1900510.
Andersen, N. I.; Serov, A.; Atanassov, P. Metal oxides/CNT nanocomposite catalysts for oxygen reduction/oxygen evolution in alkaline media. Appl. Catal. B: Environ.2015, 163, 623–627.
Li, Y. M.; He, H. Y.; Fu, W.; Mu, C. Z.; Tang, X. Z.; Liu, Z.; Chi, D. Z.; Hu, X. In-grown structure of NiFe mixed metal oxides and CNT hybrid catalysts for oxygen evolution reaction. Chem. Commun. (Camb)2016, 52, 1439–1442.
Zhang, X.; Zhang, X.; Wang, X. G.; Xie, Z. J.; Zhou, Z. NiFe2O4-CNT composite: An efficient electrocatalyst for oxygen evolution reactions in Li-O2 batteries guided by computations. J. Mater. Chem. A2016, 4, 9390–9393.
Elizabeth, I.; Nair, A. K.; Singh, B. P.; Gopukumar, S. Multifunctional Ni-NiO-CNT composite as high performing free standing anode for Li ion batteries and advanced electro catalyst for oxygen evolution reaction. Electrochim. Acta2017, 230, 98–105.
Liu, Q. H.; Cao, Q.; Bi, H.; Liang, C. Y.; Yuan, K. P.; She, W.; Yang, Y. J.; Che, R. C. CoNi@SiO2@TiO2 and CoNi@Air@TiO2 microspheres with strong wideband microwave absorption. Adv. Mater.2016, 28, 486–90.
Jiao, W. L.; Chen, C.; You, W. B.; Zhang, J.; Liu, J. W.; Che, R. C. Yolk-shell Fe/Fe4N@Pd/C magnetic nanocomposite as an efficient recyclable ORR electrocatalyst and SERS substrate. Small2019, 15, 1805032.
Li, D. J.; Kang, J.; Lee, H. J.; Choi, D. S.; Koo, S. H.; Han, B.; Kim, S. O. High activity hydrogen evolution catalysis by uniquely designed amorphous/metal interface of core-shell phosphosulfide/N-doped CNTs. Adv. Energy Mater.2018, 8, 1702806.
Xia, W.; Mahmood, A.; Liang, Z. B.; Zou, R. Q.; Guo, S. J. Earth-abundant nanomaterials for oxygen reduction. Angew. Chem., Int. Ed.2016, 55, 2650–2676.
Li, Y. F.; Selloni, A. Mechanism and activity of water oxidation on selected surfaces of pure and Fe-doped NiOx. ACS Catal.2014, 4, 1148–1153.
Hong, D. C.; Yamada, Y.; Nagatomi, T.; Takai, Y.; Fukuzumi, S. Catalysis of nickel ferrite for photocatalytic water oxidation using [Ru(bpy)3]2+ and S2O82−. J. Am. Chem. Soc.2012, 134, 19572–19575.
Kumar, P. V.; Short, M. P.; Yip, S.; Yildiz, B.; Grossman, J. C. High surface reactivity and water adsorption on NiFe2O4 (111) surfaces. J. Phys. Chem. C2013, 117, 5678–5683.
Huang, X. P.; Pan, C. X.; Huang, X. T. Preparation and characterization of γ-MnO2/CNTs nanocomposite. Mater. Lett.2007, 61, 934–936.
Yu, X. F.; Wang, L.; Liu, J. W.; Xue, S. Y.; Yang, L. T.; Li, X.; Zhang, J.; Xing, L. S.; Chen, G. Y.; Wang, M. et al. Ferromagnetic Co20Ni80 nanoparticles encapsulated inside reduced graphene oxide layers with superior microwave absorption performance. J. Mater. Chem. C2019, 7, 2943–2953.
Ye, F.; Song, Q.; Zhang, Z. C.; Li, W.; Zhang, S. Y.; Yin, X. W.; Zhou, Y. Z.; Tao, H. W.; Liu, Y. S.; Cheng, L. F. et al. Direct growth of edge-rich graphene with tunable dielectric properties in porous Si3N4 ceramic for broadband high-performance microwave absorption. Adv. Funct. Mater.2018, 28, 1707205.
Wang, H. F.; Tang, C.; Zhang, Q. A review of precious-metal-free bifunctional oxygen electrocatalysts: Rational design and applications in Zn-air batteries. Adv. Funct. Mater.2018, 28, 1803329.
Wang, C.; Han X. J.; Zhang, X. L.; Hu, S. R.; Zhang, T.; Wang, J. Y.; Du, Y. C.; Wang, X. H.; Xu, P. Controlled synthesis and morphology-dependent electromagnetic properties of hierarchical cobalt assemblies. J. Phys. Chem. C2010, 114, 14826–14830.
Wu, T.; Liu, Y.; Zeng, X.; Cui, T. T.; Zhao, Y. T.; Li, Y. N.; Tong, G. X. Facile hydrothermal synthesis of Fe3O4/C core-shell nanorings for efficient low-frequency microwave absorption. ACS Appl. Mater. Interfaces2016, 8, 7370–7380.
Chen, H.; Yan, J. Q.; Wu, H.; Zhang, Y. X.; Liu, S. Z. One-pot fabrication of NiFe2O4 nanoparticles on α-Ni(OH)2 nanosheet for enhanced water oxidation. J. Power Sources2016, 324, 499–508.
Ma, Y. D.; Dai, X. P.; Liu, M. Z.; Yong, J. X.; Qiao, H. Y.; Jin, A. X.; Li, Z. Z.; Huang, X. L.; Wang, H.; Zhang, X. Strongly coupled FeNi alloys/NiFe2O4@carbonitride layers-assembled microboxes for enhanced oxygen evolution reaction. ACS Appl. Mater. Interfaces2016, 8, 34396–34404.
Li, M.; Xiong, Y. P.; Liu, X. T.; Bo, X. J.; Zhang, Y. F.; Han, C.; Guo, L. P. Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale2015, 7, 8920–8930.
Zhao, Y.; Xu, L.; Yan, J.; Yan, W.; Wu, C. C.; Lian, J. B.; Huang, Y. P.; Bao, J.; Qiu, J. X.; Xu, L. et al. Facile preparation of NiFe2O4/MoS2 composite material with synergistic effect for high performance supercapacitor. J. Alloys Compd.2017, 726, 608–617.
Feng, S. J.; Yang, W.; Wang, Z. B. Synthesis of porous NiFe2O4 microparticles and its catalytic properties for methane combustion. Mater. Sci. Eng.: B2011, 176, 1509–1512.
Chen, L. Y.; Dai, H.; Shen, Y. M; Bai, J. F. Size-controlled synthesis and magnetic properties of NiFe2O4 hollow nanospheres via a gelassistant hydrothermal route. J. Alloys Compd.2010, 491, L33–L38.
Xu, K.; Ding, H.; Jia, K. C.; Lu, X. L.; Chen, P. Z.; Zhou, T. P.; Cheng, H.; Liu, S.; Wu, C. Z.; Xie, Y. Solution-liquid-solid synthesis of hexagonal nickel selenide nanowire arrays with a nonmetal catalyst. Angew. Chem., Int. Ed.2016, 55, 1710–1713.
Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver-nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci.2012, 258, 8807–8813.
Nesbitt, H. W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni2P XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner.2000, 27, 357–366.
Wang, X. Y.; Zhang, W. Z. Z.; Zhang, J. L.; Wu, Z. C. Fe-doped Ni3S2 nanowires with surface-restricted oxidation toward high-current-density overall water splitting. ChemElectroChem2019, 6, 4550–4559.
Wu, Z. C.; Wang, X.; Huang, J. S.; Gao, F. A Co-doped Ni-Fe mixed oxide mesoporous nanosheet array with low overpotential and high stability towards overall water splitting. J. Mater. Chem. A2018, 6, 167–178.
Wang, L. X.; Geng, J.; Wang, W. H.; Yuan, C.; Kuai, L.; Geng, B. Y. Facile synthesis of Fe/Ni bimetallic oxide solid-solution nanoparticles with superior electrocatalytic activity for oxygen evolution reaction. Nano Res.2015, 8, 3815–3822.
Mutz, B.; Sprenger, P.; Wang, W.; Wang, D.; Kleist, W.; Grunwaldt, J. D. Operando Raman spectroscopy on CO2 methanation over alumina-supported Ni, Ni3Fe and NiRh0.1 catalysts: Role of carbon formation as possible deactivation pathway. Appl. Catal. A: Gen.2018, 556, 160–171.
Li, Y. L.; Zhang, Z. Q.; Pei, L. Y.; Li, X. G.; Fan, T.; Ji, J.; Shen, J. F.; Ye, M. X. Multifunctional photocatalytic performances of recyclable Pd-NiFe2O4/reduced graphene oxide nanocomposites via different co-catalyst strategy. Appl. Catal. B: Environ.2016, 190, 1–11.
Liu, G.; Gao, X. S.; Wang, K. F.; He, D. Y.; Li, J. P. Uniformly mesoporous NiO/NiFe2O4 biphasic nanorods as efficient oxygen evolving catalyst for water splitting. Int. J. Hydrogen Energy2016, 41, 17976–17986.
Wang, X. M.; Zhang, H.; Yang, Z.; Zhang, C.; Liu, S. X. Ultrasound-treated metal-organic framework with efficient electrocatalytic oxygen evolution activity. Ultrason. Sonochem.2019, 59, 104714.
Balogun, M. S.; Qiu, W. T.; Yang, H.; Fan, W. J.; Huang, Y. C.; Fang, P. P.; Li, G. R.; Ji, H. B.; Tong, Y. X. A monolithic metal-free electrocatalyst for oxygen evolution reaction and overall water splitting. Energy Environ. Sci.2016, 9, 3411–3416.
Fang, Z. Q; Hao, Z. M.; Dong, Q. S.; Cui, Y. Bimetallic NiFe2O4 synthesized via confined carburization in NiFe-MOFs for efficient oxygen evolution reaction. J. Nanopart. Res.2018, 20, 106.
Liu, H. D.; Chen, Z. L.; Zhou, L.; Li, X.; Pei, K.; Zhang, J.; Song, Y.; Fang, F.; Che, R. C.; Sun, D. L. Rooting bismuth oxide nanosheets into porous carbon nanoboxes as a sulfur immobilizer for lithium-sulfur batteries. J. Mater. Chem. A2019, 7, 7074–7081.
Acknowledgements
This work was supported by the Ministry of Science and Technology of China (973 Project) (No. 2018YFA0209102) and the National Natural Science Foundation of China (Nos. 11727807, 51725101, 51672050, and 61790581)
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2020_2626_MOESM1_ESM.pdf
Hierarchical coupling effect in hollow Ni/NiFe2O4-CNTs microsphere via spray-drying for enhanced oxygen evolution electrocatalysis
Rights and permissions
About this article
Cite this article
Yu, X., Chen, G., Wang, Y. et al. Hierarchical coupling effect in hollow Ni/NiFe2O4-CNTs microsphere via spray-drying for enhanced oxygen evolution electrocatalysis. Nano Res. 13, 437–446 (2020). https://doi.org/10.1007/s12274-020-2626-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2626-y