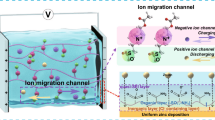
Abstract
The safe, flexible, and environment-friendly Zn-ion batteries have aroused great interests nowadays. Nevertheless, flagrant Zn dendrite uncontrollably grows in liquid electrolytes due to insufficient surface protection, which severely impedes the future applications of Zn-ion batteries especially at high current densities. Gel electrolytes are emerging to tackle this issue, yet the required high modulus for inhibiting dendrite growth as well as concurrent poor interfacial contact with roughened Zn electrodes are not easily reconcilable to regulate the fragile Zn/Zn2+ interface. Here we demonstrate, such a conflict may be defeated by using a mechanoadaptive cellulose nanofibril-based morphing gel electrolyte (MorphGE), which synergizes bulk compliance for optimizing interfacial contact as well as high modulus for suppressing dendrite formation. Moreover, by anchoring desolvated Zn2+ on cellulose nanofibrils, the side reactions which induce dendrite formation are also significantly reduced. As a result, the MorphGE-based symmetrical Zn-ion battery demonstrated outstanding stability for more than 100 h at the high current density of 10 mA·cm−2 and areal capacity of 10 mA·h·cm−2, and the corresponding Zn-ion battery delivered a prominent specific capacity of 100 mA·h·g−1 for more than 500 cycles at 20 C. The present example of engineering the mechanoadaptivity of gel electrolytes will shed light on a new pathway for designing highly safe and flexible energy storage devices.

Similar content being viewed by others
References
Pomerantseva, E.; Bonaccorso, F.; Feng, X. L.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285.
Li, Y. B.; Fu, J.; Zhong, C.; Wu, T. P.; Chen, Z. W.; Hu, W. B.; Amine, K.; Lu, J. Batteries: Recent advances in flexible zinc-based rechargeable batteries (Adv. Energy Mater. 1/2019). Adv. Energy Mater. 2019, 9, 1970001.
Ming, J.; Guo, J.; Xia, C.; Wang, W. X.; Alshareef, H. N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng.: R: Rep. 2019, 135, 58–84.
Zheng, J. X.; Zhao, Q.; Tang, T.; Yin, J. F.; Quilty, C. D.; Renderos, G. D.; Liu, X. T.; Deng, Y.; Wang, L.; Bock, D. C. et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648.
Zhu, M. S.; Wang, X. J.; Tang, H. M.; Wang, J. W.; Hao, Q.; Liu, L. X.; Li, Y.; Zhang, K.; Schmidt, O. G. Antifreezing hydrogel with high zinc reversibility for flexible and durable aqueous batteries by cooperative hydrated cations. Adv. Funct. Mater. 2020, 30, 1907218.
Mo, F. N.; Liang, G. J.; Meng, Q. Q.; Liu, Z. X.; Li, H. F.; Fan, J.; Zhi, C. Y. A flexible rechargeable aqueous zinc manganese-dioxide battery working at −20 °C. Energy Environ. Sci. 2019, 12, 706–715.
Wang, Z. Q.; Hu, J. T.; Han, L.; Wang, Z. J.; Wang, H. B.; Zhao, Q. H.; Liu, J. J.; Pan, F. A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 2019, 56, 92–99.
Leng, K. T.; Li, G. J.; Guo, J. J.; Zhang, X. Y.; Wang, A. X.; Liu, X. J.; Luo, J. Y. A safe polyzwitterionic hydrogel electrolyte for long-life quasi-solid state zinc metal batteries. Adv. Funct. Mater. 2020, 30, 2001317.
Zhao, Z. M.; Zhao, J. W.; Hu, Z. L.; Li, J. D.; Li, J. J.; Zhang, Y. J.; Wang, C.; Cui, G. L. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949.
Ma, L. T.; Chen, S. M.; Li, N.; Liu, Z. X.; Tang, Z. J.; Zapien, J. A.; Chen, S. M.; Fan, J.; Zhi, C. Y. Hydrogen-free and dendrite-dree all-solid-state Zn-ion batteries. Adv. Mater. 2020, 32, 1908121.
Yang, H. J.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X. W.; He, P.; Zhou, H. S. Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew. Chem., Int. Ed. 2020, 59, 9377–9381.
Chao, D. L.; Zhu, C.; Song, M.; Liang, P.; Zhang, X.; Tiep, N. H.; Zhao, H. F.; Wang, J.; Wang, R. M.; Zhang, H. et al. A high-rate and stable quasi-solid-state zinc-ion battery with novel 2D layered zinc orthovanadate array. Adv. Mater. 2018, 30, 1803181.
Liu, C. F.; Neale, Z.; Zheng, J. Q.; Jia, X. X.; Huang, J. J.; Yan, M. Y.; Tian, M.; Wang, M. S.; Yang, J. H.; Cao, G. Z. Expanded hydrated vanadate for high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 2273–2285.
Liu, H.; Cheng, X. B.; Huang J. Q.; Yuan, H.; Lu, Y.; Yan, C.; Zhu, G. L.; Xu, R.; Zhao, C. Z.; Hou, L. P. et al. Controlling dendrite growth in solid-state electrolytes. ACS Energy Lett. 2020, 5, 833–843.
Xia, A. L.; Pu, X. M.; Tao, Y. Y.; Liu, H. M.; Wang, Y. G. Graphene oxide spontaneous reduction and self-assembly on the zinc metal surface enabling a dendrite-free anode for long-life zinc rechargeable aqueous batteries. Appl. Surf. Sci. 2019, 481, 852–859.
Cui, Y. H.; Zhao, Q. H.; Wu, X. J.; Chen, X.; Yang, J. L.; Wang, Y. T.; Qin, R. Z.; Ding, S. X.; Song, Y. L.; Wu, J. W. et al. An interface-bridged organic-inorganic layer that suppresses dendrite formation and side reactions for ultra-long-life aqueous zinc metal anodes. Angew. Chem., Int. Ed. 2020, 59, 16594–16601.
Liu, X. Q.; Yang, F.; Xu, W.; Zeng, Y. X.; He, J. J.; Lu, X. H. Zeolitic imidazolate frameworks as Zn2+ modulation layers to enable dendrite-free Zn anodes. Adv. Sci. 2020, 7, 2002173.
Li, C.; Sun, Z. T.; Yang, T.; Yu, L. H.; Wei, N.; Tian, Z. N.; Cai, J. S.; Lv, J. Z.; Shao, Y. L.; Rümmeli, M. H. et al. Directly grown vertical graphene carpets as Janus separators toward stabilized Zn metal anodes. Adv. Mater. 2020, 32, 2003425.
Kim, J. Y.; Liu, G. C.; Shim, G. Y.; Kim, H.; Lee, J. K. Functionalized Zn@ZnO hexagonal pyramid array for dendrite-free and ultrastable zinc metal anodes. Adv. Funct. Mater. 2020, 30, 2004210.
Zhang, N. N.; Huang, S.; Yuan, Z. S.; Zhu, J. C.; Zhao, Z. F.; Niu, Z. Q. Direct self-assembly of MXene on Zn anodes for dendrite-free aqueous zinc-ion batteries. Angew. Chem., Int. Ed. 2021, 60, 2861–2865.
Li, Q.; Wang, Y. B.; Mo, F. N.; Wang, D. H.; Liang, G. J.; Zhao, Y. W.; Yang, Q.; Huang, Z. D.; Zhi, C. Y. Calendar life of Zn batteries based on Zn anode with Zn powder/current collector structure. Adv. Energy Mater. 2021, 11, 2003931.
Xu, W. N.; Zhao, K. N.; Huo, W. C.; Wang, Y. Z.; Yao, G.; Gu, X.; Cheng, H. W.; Mai, L.; Hu, C. G.; Wang, X. D. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy 2019, 62, 275–281.
Zhao, J. W.; Zhang, J.; Yang, W. H.; Chen, B. B.; Zhao, Z. M.; Qiu, H. Y.; Dong, S. M.; Zhou, X. H.; Cui, G. L.; Chen, L. Q. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 2019, 57, 625–634.
Chang, N. N.; Li, T. Y.; Li, R.; Wang, S. N.; Yin, Y. B.; Zhang, H. M.; Li, X. F. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ. Sci. 2020, 13, 3527–3535.
Cao, L. S.; Li, D.; Hu, E. Y.; Xu, J. J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X. Q.; Wang, C. S. Solvation structure design for aqueous Zn metal batteries. J. Am. Chem. Soc. 2020, 142, 21404–21409.
Yuan, D.; Zhao, J.; Ren, H.; Chen, Y. Q.; Chua, R.; Jie, E. T. J.; Cai, Y.; Edison, E.; Manalastas, W. Jr.; Wong, M. W. et al. Anion texturing towards dendrite-free Zn anode for aqueous rechargeable batteries. Angew. Chem., Int. Ed. 2021, 60, 7213–7219.
Mo, F. N.; Chen, Z.; Liang, G. J.; Wang, D. H.; Zhao, Y. W.; Li, H. F.; Dong, B. B.; Zhi, C. Y. Zwitterionic sulfobetaine hydrogel electrolyte building separated positive/negative ion migration channels for aqueous Zn-MnO2 batteries with superior rate capabilities. Adv. Energy Mater. 2020, 10, 2000035.
Qiu, H. Y.; Du, X. F.; Zhao, J. W.; Wang, Y. T.; Ju, J. W.; Chen, Z.; Hu, Z. L.; Yan, D. P.; Zhou, X. H.; Cui, G. L. Zinc anode-compatible in-situ solid electrolyte interphase via cation solvation modulation. Nat. Commun. 2019, 10, 5374.
Li, H. F.; Han, C. P.; Huang, Y.; Huang, Y.; Zhu, M. S.; Pei, Z. X.; Xue, Q.; Wang, Z. F.; Liu, Z. X.; Tang, Z. J. et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 2018, 11, 941–951.
Zhang, Q. C.; Li, C. W.; Li, Q. L.; Pan, Z. H.; Sun, J.; Zhou, Z. Y.; He, B.; Man, P.; Xie, L. Y.; Kang, L. X. et al. Flexible and high-voltage coaxial-fiber aqueous rechargeable zinc-ion battery. Nano Lett. 2019, 19, 4035–4042.
Li, H. F.; Liu, Z. X.; Liang, G. J.; Huang, Y.; Huang, Y.; Zhu, M. S.; Pei, Z. X.; Xue, Q.; Tang, Z. J.; Wang, Y. K. et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 2018, 12, 3140–3148.
Han, Q.; Chi, X. W.; Zhang, S. M.; Liu, Y. Z.; Zhou, B.; Yang, J. H.; Liu, Y. Durable, flexible self-standing hydrogel electrolytes enabling high-safety rechargeable solid-state zinc metal batteries. J. Mater. Chem. A 2018, 6, 23046–23054.
Huang, S.; Wan, F.; Bi, S. S.; Zhu, J. C.; Niu, Z. Q.; Chen, J. A self-healing integrated all-in-one zinc-ion battery. Angew. Chem., Int. Ed. 2019, 58, 4313–4317.
Wu, H. P.; Cao, Y.; Su, H. P.; Wang, C. Tough gel electrolyte using double polymer network design for the safe, stable cycling of lithium metal anode. Angew. Chem., Int. Ed. 2018, 57, 1361–1365.
Ye, F.; Zhang, X.; Liao, K. M.; Lu, Q.; Zou, X. H.; Ran, R.; Zhou, W.; Zhong, Y. J.; Shao, Z. P. A smart lithiophilic polymer filler in gel polymer electrolyte enables stable and dendrite-free Li metal anode. J. Mater. Chem. A 2020, 8, 9733–9742.
Wang, D. H.; Li, H. F.; Liu, Z. X.; Tang, Z. J.; Liang, G. J.; Mo, F. N.; Yang, Q.; Ma, L. T.; Zhi, C. Y. A nanofibrillated cellulose/polyacrylamide electrolyte-based flexible and sewable high-performance Zn-MnO2 battery with superior shear resistance. Small 2018, 14, 1803978.
Liu, K.; Pei, A.; Lee, H. R.; Kong, B.; Liu, N.; Lin, D. C.; Liu, Y. Y.; Liu, C.; Hsu, P. C.; Bao, Z. N. et al. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 2017, 139, 4815–4820.
Luo, Y. F.; Li, W. L.; Lin, Q. Y.; Zhang, F. L.; He, K.; Yang, D. P.; Loh, X. J.; Chen, X. D. A morphable ionic electrode based on thermogel for non-invasive hairy plant electrophysiology. Adv. Mater. 2021, 33, 2007848.
Liu, Y. X.; Li, J. X.; Song, S.; Kang, J.; Tsao, Y.; Chen, S. C.; Mottini, V.; McConnell, K.; Xu, W. H.; Zheng, Y. Q. et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 2020, 38, 1031–1036.
Zhang, X. T.; Wu, B. H.; Sun, S. T.; Wu, P. Y. Hybrid materials from ultrahigh-inorganic-content mineral plastic hydrogels: Arbitrarily shapeable, strong, and tough. Adv. Funct. Mater. 2020, 30, 1910425.
Cao, Z. Y.; Zhuang, P. Y.; Zhang, X.; Ye, M. X.; Shen, J. F.; Ajayan, P. M. Strategies for dendrite-free anode in aqueous rechargeable zinc ion batteries. Adv. Energy Mater. 2020, 10, 2001599.
Mredha, M. T. I.; Le, H. H.; Tran, V. T.; Trtik, P.; Cui, J. X.; Jeon, I. Anisotropic tough multilayer hydrogels with programmable orientation. Mater. Horiz. 2019, 6, 1504–1511.
Pedersen, J. S.; Schurtenberger, P. Scattering functions of semiflexible polymers with and without excluded volume effects. Macromolecules 1996, 29, 7602–7612.
Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T. M.; Janaswamy, S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281.
Zhang, X. F.; Hou, T.; Chen, J.; Feng, Y.; Li, B. G.; Gu, X. L.; He, M.; Yao, J. F. Facilitated transport of CO2 through the transparent and flexible cellulose membrane promoted by fixed-site carrier. ACS Appl. Mater. Interfaces 2018, 10, 24930–24936.
Zhang, X. F.; Ma, X. F.; Hou, T.; Guo, K. C.; Yin, J. Y.; Wang, Z. G.; Shu, L.; He, M.; Yao, J. F. Inorganic salts induce thermally reversible and anti-freezing cellulose hydrogels. Angew. Chem., Int. Ed. 2019, 58, 7366–7370.
Cong, J. L.; Shen, X.; Wen, Z. P.; Wang, X.; Peng, L. Q.; Zeng, J.; Zhao, J. B. Ultra-stable and highly reversible aqueous zinc metal anodes with high preferred orientation deposition achieved by a polyanionic hydrogel electrolyte. Energy Stor. Mater. 2021, 35, 586–594.
Hao, J. N.; Li, X. L.; Zhang, S. L.; Yang, F. H.; Zeng, X. H.; Zhang, S.; Bo, G. Y.; Wang, C. S.; Guo, Z. P. Designing dendrite-free zinc anodes for advanced aqueous zinc batteries. Adv. Funct. Mater. 2020, 30, 2001263.
Qin, R. Z.; Wang, Y. T.; Zhang, M. Z.; Wang, Y.; Ding, S. X.; Song, A. Y.; Yi, H. C.; Yang, L. Y.; Song, Y. L.; Cui, Y. H. et al. Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 2021, 80, 105478.
Zeng, X. H.; Liu, J. T.; Mao, J. F.; Hao, J. N.; Wang, Z. J.; Zhou, S.; Ling, C. D.; Guo, Z. P. Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry. Adv. Energy Mater. 2020, 10, 1904163.
Cui, M. W.; Xiao, Y.; Kang, L. T.; Du, W.; Gao, Y. F.; Sun, X. Q.; Zhou, Y. L.; Li, X. M.; Li, H. F.; Jiang, F. Y. et al. Quasi-isolated Au particles as heterogeneous seeds to guide uniform Zn deposition for aqueous zinc-ion batteries. ACS Appl. Energy Mater. 2019, 2, 6490–6496.
Dong, L. B.; Yang, W.; Yang, W.; Tian, H.; Huang, Y. F.; Wang, X. L.; Xu, C. J.; Wang, C. Y.; Kang, F. Y.; Wang, G. X. Flexible and conductive scaffold-stabilized zinc metal anodes for ultralong-life zinc-ion batteries and zinc-ion hybrid capacitors. Chem. Eng. J. 2020, 384, 123355.
Zeng, Y. X.; Zhang, X. Y.; Qin, R. F.; Liu, X. Q.; Fang, P. P.; Zheng, D. Z.; Tong, Y. X.; Lu, X. H. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv. Mater. 2019, 31, 1903675.
Ma, L. T.; Chen, S. M.; Li, X. L.; Chen, A.; Dong, B. B.; Zhi, C. Y. Liquid-free All-Solid-State zinc batteries and encapsulation-free flexible batteries enabled by in situ constructed polymer electrolyte. Angew. Chem., Int. Ed. 2020, 59, 23836–23844.
Song, Z. S.; Ding, J.; Liu, B.; Liu, X. R.; Han, X. P.; Deng, Y. D.; Hu, W. B.; Zhong, C. A rechargeable Zn-air battery with high energy efficiency and long life enabled by a highly water-retentive gel electrolyte with reaction modifier. Adv. Mater. 2020, 32, 1908127.
Acknowledgements
This work was supported by the National Science Foundation of China (NSFC) (Nos. 51903041, 21991123, and 51873035), Natural Science Foundation of Shanghai (No. 19ZR1470700), and “Qimingxing Plan” (No. 19QA1400200). The authors thank the staffs from BL16B beamline at Shanghai Synchrotron Radiation Facility for assistance during data collection.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_3770_MOESM1_ESM.pdf
Mechanoadaptive morphing gel electrolyte enables flexible and fast-charging Zn-ion batteries with outstanding dendrite suppression performance
Rights and permissions
About this article
Cite this article
Cao, F., Wu, B., Li, T. et al. Mechanoadaptive morphing gel electrolyte enables flexible and fast-charging Zn-ion batteries with outstanding dendrite suppression performance. Nano Res. 15, 2030–2039 (2022). https://doi.org/10.1007/s12274-021-3770-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3770-8