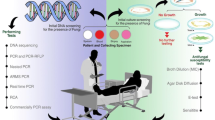
Abstract
Purpose of Review
The incidence of invasive aspergillosis has increased substantially over the past few decades, accompanied by a change in susceptibility patterns of Aspergillus fumigatus with increasing resistance observed against triazole antifungals, including voriconazole and isavuconazole, the most commonly used antifungal agents for the disease. Culture-based methods for determining triazole resistance are still the gold standard but are time consuming and lack sensitivity. We sought to provide an update on non-culture-based methods for detecting resistance patterns to Aspergillus.
Recent Findings
New molecular-based approaches for detecting triazole resistance to Aspergillus, real-time polymerase chain reaction (PCR) to detect mutations to the Cyp51A protein, have been developed which are able to detect most triazole-resistant A. fumigatus strains in patients with invasive aspergillosis.
Summary
Over the last few years, a number of non-culture-based methods for molecular detection of Aspergillus triazole resistance have been developed that may overcome some of the limitations of culture. These molecular methods are therefore of high epidemiological and clinical relevance, mainly in immunocompromised patients with hematological malignancies, where culture has particularly limited sensitivity. These assays are now able to detect most triazole-resistant Aspergillus fumigatus strains. Given that resistance rates vary, clinical utility for these assays still depends on regional resistance patterns.
Similar content being viewed by others
Change history
10 July 2019
Funding information was incomplete. The correct information is given below.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Hoenigl M, Gangneux JP, Segal E, Alanio A, Chakrabarti A, Chen SC, et al. Global guidelines and initiatives from the European Confederation of Medical Mycology to improve patient care and research worldwide: new leadership is about working together. Mycoses. 2018;61(11):885–94. https://doi.org/10.1111/myc.12836 Describes global guidelines and optimal collaboration strategies to optimize clinical care in resource-rich and resource-limited settings.
•• Jenks JD, Mehta SR, Taplitz R, Aslam S, Reed SL, Hoenigl M. Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test in bronchoalveolar lavage. Mycoses. 2019;62(3):230–6. https://doi.org/10.1111/myc.12881 Paper highlights the difficulties of defining, categorizing, and diagnosing Invasive aspergillosis in non-neutropenic hosts.
Cornely OA, Lass-Florl C, Lagrou K, Arsic-Arsenijevic V, Hoenigl M. Improving outcome of fungal diseases - guiding experts and patients towards excellence. Mycoses. 2017. https://doi.org/10.1111/myc.12628.
Heldt S, Prattes J, Eigl S, Spiess B, Flick H, Rabensteiner J, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Infect. 2018;77(3):235–41. https://doi.org/10.1016/j.jinf.2018.05.001.
• Hoenigl M, Orasch T, Faserl K, Prattes J, Loeffler J, Springer J, et al. Triacetylfusarinine C: a urine biomarker for diagnosis of invasive aspergillosis. J Infect. 2019;78(2):150–7. https://doi.org/10.1016/j.jinf.2018.09.006 Important paper, as point-of-care diagnostics play a major role in critically ill patients.
Jenks JD, Mehta SR, Taplitz R, Law N, Reed SL, Hoenigl M. Bronchoalveolar lavage Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test for diagnosis of invasive pulmonary aspergillosis in patients with hematological malignancies. The Journal of infection. 2019;78(3):249–59. https://doi.org/10.1016/j.jinf.2018.10.014.
• Jenks JD, Salzer HJF, Hoenigl M. Improving the rates of Aspergillus detection: an update on current diagnostic strategies. Expert Rev Anti-Infect Ther. 2019;17(1):39–50. https://doi.org/10.1080/14787210.2018.1558054 Diagnosis of fungal infections is a major issue in the treatment of immunocompromised patients. This review provides an important overview on the current status of diagnostic strategies.
Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: which drug and when? Med Mycol. 2019;57(Supplement_2):S168–s78. https://doi.org/10.1093/mmy/myy052.
Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62(3):362–8. https://doi.org/10.1093/cid/civ885.
•• Schauwvlieghe A, de Jonge N, van Dijk K, Verweij PE, Bruggemann RJ, Biemond BJ, et al. The diagnosis and treatment of invasive aspergillosis in Dutch haematology units facing a rapidly increasing prevalence of azole-resistance. A nationwide survey and rationale for the DB-MSG 002 study protocol. Mycoses. 2018;61(9):656–64. https://doi.org/10.1111/myc.12788 This paper describes the increasing incidence of azole resistance in the Netherlands, an increasing problem globally.
Eigl S, Hoenigl M, Spiess B, Heldt S, Prattes J, Neumeister P, et al. Galactomannan testing and Aspergillus PCR in same-day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med Mycol. 2017;55(5):528–34. https://doi.org/10.1093/mmy/myw102.
Hoenigl M. Fungal translocation: a driving force behind the occurrence of non-AIDS events? Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz215.
Hoenigl M, Eigl S, Heldt S, Duettmann W, Thornton C, Prattes J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses. 2018;61(1):40–3. https://doi.org/10.1111/myc.12704.
Postina P, Skladny J, Boch T, Cornely OA, Hamprecht A, Rath PM, et al. Comparison of two molecular assays for detection and characterization of Aspergillus fumigatus triazole resistance and Cyp51A mutations in clinical isolates and primary clinical samples of immunocompromised patients. Front Microbiol. 2018;9:555. https://doi.org/10.3389/fmicb.2018.00555.
White PL, Perry MD, Moody A, Follett SA, Morgan G, Barnes RA. Evaluation of analytical and preliminary clinical performance of Myconostica MycAssay Aspergillus when testing serum specimens for diagnosis of invasive aspergillosis. J Clin Microbiol. 2011;49(6):2169–74. https://doi.org/10.1128/jcm.00101-11.
White PL, Hibbitts SJ, Perry MD, Green J, Stirling E, Woodford L, et al. Evaluation of a commercially developed semiautomated PCR-surface-enhanced Raman scattering assay for diagnosis of invasive fungal disease. J Clin Microbiol. 2014;52(10):3536–43. https://doi.org/10.1128/jcm.01135-14.
Torelli R, Sanguinetti M, Moody A, Pagano L, Caira M, De Carolis E, et al. Diagnosis of invasive aspergillosis by a commercial real-time PCR assay for Aspergillus DNA in bronchoalveolar lavage fluid samples from high-risk patients compared to a galactomannan enzyme immunoassay. J Clin Microbiol. 2011;49(12):4273–8. https://doi.org/10.1128/jcm.05026-11.
Chong GL, van de Sande WW, Dingemans GJ, Gaajetaan GR, Vonk AG, Hayette MP, et al. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol. 2015;53(3):868–74. https://doi.org/10.1128/jcm.03216-14.
Huttner A, Emonet S, Harbarth S, Renzi G, Kaiser L, Schrenzel J. Polymerase-chain reaction/electrospray ionization-mass spectrometry for the detection of bacteria and fungi in bronchoalveolar lavage fluids: a prospective observational study. Clin Microbiol Infect. 2014;20(12):O1059–66. https://doi.org/10.1111/1469-0691.12749.
Klaassen CH, de Valk HA, Curfs-Breuker IM, Meis JF. Novel mixed-format real-time PCR assay to detect mutations conferring resistance to triazoles in Aspergillus fumigatus and prevalence of multi-triazole resistance among clinical isolates in the Netherlands. J Antimicrob Chemother. 2010;65(5):901–5. https://doi.org/10.1093/jac/dkq041.
Verweij PE, Mellado E, Melchers WJ. Multiple-triazole-resistant aspergillosis. N Engl J Med. 2007;356(14):1481–3. https://doi.org/10.1056/NEJMc061720.
Price CL, Parker JE, Warrilow AG, Kelly DE, Kelly SL. Azole fungicides - understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci. 2015;71(8):1054–8. https://doi.org/10.1002/ps.4029.
Azevedo MM, Faria-Ramos I, Cruz LC, Pina-Vaz C, Rodrigues AG. Genesis of azole antifungal resistance from agriculture to clinical settings. J Agric Food Chem. 2015;63(34):7463–8. https://doi.org/10.1021/acs.jafc.5b02728.
Pfaller MA, Rhomberg PR, Wiederhold NP, Gibas C, Sanders C, Fan H, et al. In vitro activity of isavuconazole against opportunistic fungal pathogens from two mycology reference laboratories. Antimicrob Agents Chemother. 2018;62(10). https://doi.org/10.1128/aac.01230-18.
Pinto E, Monteiro C, Maia M, Faria MA, Lopes V, Lameiras C, et al. Aspergillus species and antifungals susceptibility in clinical setting in the north of Portugal: cryptic species and emerging azoles resistance in A. fumigatus. Front Microbiol. 2018;9:1656. https://doi.org/10.3389/fmicb.2018.01656.
Buil JB, Bruggemann RJM, Wasmann RE, Zoll J, Meis JF, Melchers WJG, et al. Isavuconazole susceptibility of clinical Aspergillus fumigatus isolates and feasibility of isavuconazole dose escalation to treat isolates with elevated MICs. J Antimicrob Chemother. 2018;73(1):134–42. https://doi.org/10.1093/jac/dkx354.
Institute. CaLS. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi.; Approved standard. In: CLSI document M38. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
Espinel-Ingroff A. Comparison of three commercial assays and a modified disk diffusion assay with two broth microdilution reference assays for testing zygomycetes, Aspergillus spp., Candida spp., and Cryptococcus neoformans with posaconazole and amphotericin B. J Clin Microbiol. 2006;44(10):3616–22. https://doi.org/10.1128/jcm.01187-06.
Martin-Mazuelos E, Peman J, Valverde A, Chaves M, Serrano MC, Canton E. Comparison of the Sensititre YeastOne colorimetric antifungal panel and Etest with the NCCLS M38-A method to determine the activity of amphotericin B and itraconazole against clinical isolates of Aspergillus spp. J Antimicrob Chemother. 2003;52(3):365–70. https://doi.org/10.1093/jac/dkg384.
Pfaller MA, Messer SA, Boyken L, Hollis RJ, Diekema DJ. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference M38-A microdilution methods for determining posaconazole MICs. Diagn Microbiol Infect Dis. 2003;45(4):241–4.
Araujo R, Espinel-Ingroff A. Comparison of assessment of oxygen consumption, Etest, and CLSI M38-A2 broth microdilution methods for evaluation of the susceptibility of Aspergillus fumigatus to posaconazole. Antimicrob Agents Chemother. 2009;53(11):4921–3. https://doi.org/10.1128/aac.00862-09.
Arendrup MC, Verweij P, Nielsen HV. Evaluation of MIC strip isavuconazole test for susceptibility testing of wild-type and non-wild-type Aspergillus fumigatus isolates. Antimicrob Agents Chemother. 2017;61(1). https://doi.org/10.1128/aac.01659-16.
Jenks JD, Salzer HJ, Prattes J, Krause R, Buchheidt D, Hoenigl M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:1033–44. https://doi.org/10.2147/DDDT.S145545.
Espinel-Ingroff A, Rezusta A. E-test method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J Clin Microbiol. 2002;40(6):2101–7.
Fuller J, Schofield A, Jiwa S, Sand C, Jansen B, Rennie R. Caspofungin Etest endpoint for Aspergillus isolates shows poor agreement with the reference minimum effective concentration. J Clin Microbiol. 2010;48(2):479–82. https://doi.org/10.1128/jcm.01677-09.
Espinel-Ingroff A, Turnidge J, Alastruey-Izquierdo A, Botterel F, Canton E, Castro C, et al. Method-dependent epidemiological cutoff values for detection of triazole resistance in Candida and Aspergillus species for the Sensititre YeastOne colorimetric broth and Etest agar diffusion methods. Antimicrob Agents Chemother. 2019;63(1). https://doi.org/10.1128/aac.01651-18.
Guinea J, Verweij PE, Meletiadis J, Mouton JW, Barchiesi F, Arendrup MC. How to: EUCAST recommendations on the screening procedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four-well azole-containing agar plates. Clin Microbiol Infect. 2018. https://doi.org/10.1016/j.cmi.2018.09.008.
Arnold RJ, Reilly JP. Fingerprint matching of E. coli strains with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of whole cells using a modified correlation approach. Rapid Commun Mass Spectrom : RCM. 1998;12(10):630–6. https://doi.org/10.1002/(sici)1097-0231(19980529)12:10<630::Aid-rcm206>3.0.Co;2-0.
Marinach C, Alanio A, Palous M, Kwasek S, Fekkar A, Brossas JY, et al. MALDI-TOF MS-based drug susceptibility testing of pathogens: the example of Candida albicans and fluconazole. Proteomics. 2009;9(20):4627–31. https://doi.org/10.1002/pmic.200900152.
De Carolis E, Vella A, Florio AR, Posteraro P, Perlin DS, Sanguinetti M, et al. Use of matrix-assisted laser desorption ionization-time of flight mass spectrometry for caspofungin susceptibility testing of Candida and Aspergillus species. J Clin Microbiol. 2012;50(7):2479–83. https://doi.org/10.1128/jcm.00224-12.
Gitman MR, McTaggart L, Spinato J, Poopalarajah R, Lister E, Husain S, et al. Antifungal susceptibility testing of Aspergillus spp. by using a composite correlation index (CCI)-based matrix-assisted laser desorption ionization-time of flight mass spectrometry method appears to not offer benefit over traditional broth microdilution testing. J Clin Microbiol. 2017;55(7):2030–4. https://doi.org/10.1128/jcm.00254-17.
Rizzato C, Lombardi L, Zoppo M, Lupetti A, Tavanti A. Pushing the limits of MALDI-TOF mass spectrometry: beyond fungal species identification. J Fungi (Basel, Switzerland). 2015;1(3):367–83. https://doi.org/10.3390/jof1030367.
Sanguinetti M, Posteraro B. Identification of molds by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2017;55(2):369–79. https://doi.org/10.1128/jcm.01640-16.
Spiess B, Postina P, Reinwald M, Cornely OA, Hamprecht A, Hoenigl M, et al. Incidence of Cyp51 A key mutations in Aspergillus fumigatus-a study on primary clinical samples of immunocompromised patients in the period of 1995-2013. PLoS One. 2014;9(7):e103113. https://doi.org/10.1371/journal.pone.0103113.
Spiess B, Seifarth W, Merker N, Howard SJ, Reinwald M, Dietz A, et al. Development of novel PCR assays to detect azole resistance-mediating mutations of the Aspergillus fumigatus cyp51A gene in primary clinical samples from neutropenic patients. AntimicrobAgents Chemother. 2012;56(7):3905–10.
Perfect JR, Hachem R, Wingard JR. Update on epidemiology of and preventive strategies for invasive fungal infections in cancer patients. Clin Infect Dis. 2014;59(Suppl 5):S352–5. https://doi.org/10.1093/cid/ciu639.
Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, et al. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70(5):1522–6. https://doi.org/10.1093/jac/dku566.
Chong GM, van der Beek MT, von dem Borne PA, Boelens J, Steel E, Kampinga GA, et al. PCR-based detection of Aspergillus fumigatus Cyp51A mutations on bronchoalveolar lavage: a multicentre validation of the AsperGenius assay(R) in 201 patients with haematological disease suspected for invasive aspergillosis. J Antimicrob Chemother. 2016;71:3528–35. https://doi.org/10.1093/jac/dkw323.
•• Koehler P, Hamprecht A, Bader O, Bekeredjian-Ding I, Buchheidt D, Doelken G, et al. Epidemiology of invasive aspergillosis and azole resistance in patients with acute leukaemia: the SEPIA Study. Int J Antimicrob Agents. 2017;49(2):218–23. https://doi.org/10.1016/j.ijantimicag.2016.10.019 This very important study shows the clinical relevance of azole resistance in hematological patients on triazole therapy with azole-resistant A. fumigatus infections.
Trama JP, Mordechai E, Adelson ME. Detection of Aspergillus fumigatus and a mutation that confers reduced susceptibility to itraconazole and posaconazole by real-time PCR and pyrosequencing. J Clin Microbiol. 2005;43(2):906–8. https://doi.org/10.1128/jcm.43.2.906-908.2005.
van der Linden JW, Snelders E, Arends JP, Daenen SM, Melchers WJ, Verweij PE. Rapid diagnosis of azole-resistant aspergillosis by direct PCR using tissue specimens. JClinMicrobiol. 2010;48(4):1478–80.
Denning DW, Park S, Lass-Florl C, Fraczek MG, Kirwan M, Gore R, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. ClinInfectDis. 2011;52(9):1123–9.
Weber M, Schaer J, Walther G, Kaerger K, Steinmann J, Rath PM, et al. FunResDB-A web resource for genotypic susceptibility testing of Aspergillus fumigatus. Med Mycol. 2018;56(1):117–20. https://doi.org/10.1093/mmy/myx015.
•• Rath PM, Steinmann J. Overview of commercially available PCR assays for the detection of Aspergillus spp. DNA in patient samples. Front Microbiol. 2018;9:740. https://doi.org/10.3389/fmicb.2018.00740 This paper compares the five most important commercially available molecular Aspergillus DNA and azole-resistant detection systems.
Schauwvlieghe A, Vonk AG, Buddingh EP, Hoek RAS, Dalm VA, Klaassen CHW, et al. Detection of azole-susceptible and azole-resistant Aspergillus coinfection by cyp51A PCR amplicon melting curve analysis. J Antimicrob Chemother. 2017;72(11):3047–50. https://doi.org/10.1093/jac/dkx262.
White PL, Barnes RA, Springer J, Klingspor L, Cuenca-Estrella M, Morton CO, et al. The clinical performance of Aspergillus PCR when testing serum and plasma- a study by the European Aspergillus PCR Initiative. J Clin Microbiol. 2015;53:2832–7. https://doi.org/10.1128/jcm.00905-15.
White PL, Posso RB, Barnes RA. Analytical and clinical evaluation of the PathoNostics AsperGenius assay for detection of invasive aspergillosis and resistance to azole antifungal drugs directly from plasma samples. J Clin Microbiol. 2017;55(8):2356–66. https://doi.org/10.1128/jcm.00411-17.
Dannaoui E, Gabriel F, Gaboyard M, Lagardere G, Audebert L, Quesne G, et al. Molecular diagnosis of invasive aspergillosis and detection of azole resistance by a newly commercialized PCR kit. J Clin Microbiol. 2017;55(11):3210–8. https://doi.org/10.1128/jcm.01032-17.
Guegan H, Chevrier S, Belleguic C, Deneuville E, Robert-Gangneux F, Gangneux JP. Performance of molecular approaches for Aspergillus detection and azole resistance surveillance in cystic fibrosis. Front Microbiol. 2018;9:531. https://doi.org/10.3389/fmicb.2018.00531.
Tsitsopoulou A, Posso R, Vale L, Bebb S, Johnson E, White PL. Determination of the prevalence of triazole resistance in environmental Aspergillus fumigatus strains isolated in South Wales, UK. Front Microbiol. 2018;9:1395. https://doi.org/10.3389/fmicb.2018.01395.
Zoran T, Sartori B, Sappl L, Aigner M, Sanchez-Reus F, Rezusta A, et al. Azole-resistance in Aspergillus terreus and related species: an emerging problem or a rare phenomenon? Front Microbiol. 2018;9:516. https://doi.org/10.3389/fmicb.2018.00516.
Buil JB, Zoll J, Verweij PE, Melchers WJG. Molecular detection of azole-resistant Aspergillus fumigatus in clinical samples. Front Microbiol. 2018;9:515. https://doi.org/10.3389/fmicb.2018.00515.
Funding
This work was supported using funds from the Oesterreichische Nationalbank (Anniversary Fund, project number 15346).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclaimer
The funders had no role in the study design, data collection, analysis, interpretation, decision to publish, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Conflict of Interest
Dieter Buchheidt reports being a consultant to Basilea, Gilead Sciences, and Merck Sharp & Dohme/Merck; receiving research grants from Gilead Sciences and Pfizer; serving on the speakers’ bureau of Astellas, Basilea, Gilead Sciences, Merck Sharp & Dohme/Merck, Pfizer, and TEVA; and receiving travel grants from Astellas, Gilead Sciences, Merck Sharp & Dohme/Merck, and Pfizer. Martin Hoenigl reports untied research funding from Gilead. Jeffrey Jenks and Birgit Spiess declare no conflict of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jenks, J.D., Spiess, B., Buchheidt, D. et al. (New) Methods for Detection of Aspergillus fumigatus Resistance in Clinical Samples. Curr Fungal Infect Rep 13, 129–136 (2019). https://doi.org/10.1007/s12281-019-00342-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-019-00342-w