Abstract
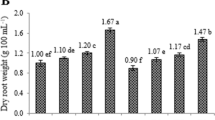
Ajuga bracteosa is a medicinally important plant globally used in the folk medicine against many serious ailments. In the present study, effects of two significant elicitors, methyl jasmonate (Me-J) and phenyl acetic acid (PAA) were studied on growth parameters, secondary metabolites production, and antioxidant potential in adventitious root suspension cultures of A. bracteosa. The results showed a substantial increase in biomass accumulation, exhibiting longer log phases of cultures growth in response to elicitor treatments, in comparison to control. Maximum dry biomass formation (8.88 DW g/L) was recorded on 32nd day in log phase of culture when 0.6 mg/L Me-J was applied; however, PAA at 1.2 mg/L produced maximum biomass (8.24 DW g/L) on day 40 of culture. Furthermore, we observed the elicitors-induced enhancement in phenolic content (total phenolic content), flavonoid content (total flavonoid content) and antioxidant activity (free radical scavenging activity) in root suspension cultures of A. bracteosa. Application of 0.6 mg/L and 1.2 mg/L of Me-J, root cultures accumulated higher TPC levels (3.6 mg GAE/g DW) and (3.7 mg GAE/g DW) in the log phase and stationary phase, respectively, while 2.5 mg/L Me-J produced lower levels (1.4 mg GAE/g DW) in stationary phase of growth stages. Moreover, TFC and FRSA values were found in correspondence to TPC values in the respective growth phases at the similar elicitor treatment. Thus, a feasible protocol for establishment of adventitious roots in A. bracteosa was developed and enhancement in biomass and metabolite content in adventitious root was promoted through elicitation.




Similar content being viewed by others
References
Abbasi BH, Khan MA, Mahmood T, Ahmad M, Chaudhary MF, Khan MA (2010) Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult (PCTOC) 101:371–376. doi:10.1007/s11240-010-9692-x
Abbasi BH, Ali H, Yücesan B, Saeed S, Rehman K, Khan MA (2016) Evaluation of biochemical markers during somatic embryogenesis in Silybum marianum L. 3 Biotech 6:1–8. doi:10.1007/s13205-016-0366-1
Ali M, Hahn E-J, Paek K-Y (2007) Methyl Jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12:607
Arfan M, Khan GA, Ahmad N (1996) Steroids and terpenoids of the genus Ajuga. J Chem Soc Pak 18:170–174
Bathoju G, Giri A (2012) Production of medicinally important secondary metabolites (stigmasterol and hecogenin) from root cultures of Chlorophytum borivilianum (Safed musli). Recent Res Sci Technol 4:45–48
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10(3):178–182
Chopra RN, Chopra IC, Nayar SL (1956) Glossary of Indian medicinal plants. Council of Scientific and Industrial Research, New Delhi
De Klerk G-J, Brugge JT, Marinova S (1997) Effectiveness of indoleacetic acid, indolebutyric acid and naphthaleneacetic acid during adventitious root formation in vitro in Malus ‘Jork 9’. Plant Cell Tissue Organ Cult 49:39–44. doi:10.1023/a:1005850222973
Dinan L (2001) Phytoecdysteroids: biological aspects. Phytochemistry 57:325–339. doi:10.1016/S0031-9422(01)00078-4
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085
Dunlap JR, Kresovich S, McGee RE (1986) The effect of salt concentration on auxin stability in culture media. Plant Physiol 81:934–936
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Gautam R, Jachak SM, Saklani A (2011) Anti-inflammatory effect of Ajuga bracteosa Wall. ex Benth. mediated through cyclooxygenase (COX) inhibition. J Ethnopharmacol 133:928–930
Gundlach H, Muller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA 89:2389–2393
Heller W, Forkmann G (1988) Biosynthesis. In: Harborne JB, Mabry TJ, Mabry H (eds) The flavonoids. Springer, pp 399–425
Jalalpour Z, Shabani L, Afghani L, Sharifi-Tehrani M, Amini S-A (2014) Stimulatory effect of Methyl Jasmonate and squalestatin on phenolic metabolism through induction of LOX activity in cell suspension culture of yew. Turk J Biol 38:76–82
Jan M, Singh S, Kaloo ZA, Maqbool F (2014) Callus induction and multiple shoot regeneration in Ajuga bracteosa Wall. ex Benth.—an important medicinal plant growing in Kashmir Himalaya. J Sci Innov Res 3:319–324
Kayani WK, Rani R, Ihsan-ul H, Mirza B (2014) Seasonal and geographical impact on the morphology and 20-hydroxyecdysone content in different tissue types of wild Ajuga bracteosa Wall. ex Benth. Steroids 87:12–20. doi:10.1016/j.steroids.2014.04.017
Khan MA, Abbasi BH, Ahmed N, Ali H (2013) Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Ind Crops Prod 46:105–110
Khan MA, Abbasi BH, Ali H, Ali M, Adil M, Hussain I (2015a) Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Silybum marianum L. Plant Cell Tissue Organ Cult (PCTOC) 120:127–139
Khan MA, Abbasi BH, Shah NA, Yücesan B, Ali H (2015b) Analysis of metabolic variations throughout growth and development of adventitious roots in Silybum marianum L. (Milk thistle), a medicinal plant. Plant Cell Tissue Organ Cult (PCTOC) 123:501–510. doi:10.1007/s11240-015-0854-8
Lee YS, Yang T-J, Park SU, Baek JH, Wu SQ, Lim K-B (2011) Induction and proliferation of adventitious roots from ‘Aloe vera’ leaf tissues for ‘in vitro’ production of aloe-emodin. Plant Omics 4:190
Lister CE, Lancaster JE, Walker JR (1996) Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J Sci Food Agric 71:313–320
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2 −, H2O2, and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123. doi:10.1104/pp.104.044784
Misico RI, Nicotra VE, Oberti JC, Barboza G, Gil RR, Burton G (2011) Withanolides and related steroids. In: Progress in the chemistry of organic natural products, vol 94. Springer, pp 127–229
Mulabagal V, Tsay H-S (2004) Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Paek K-Y, Hahn E-J, Son S-H (2001) Application of bioreactors for large-scale micropropagation systems of plants. In Vitro Cell Dev Biol Plant 37:149–157
Pala A et al (2011) Ajuga bracteosa wall: a review on its ethnopharmacological and phytochemical studies. Der Pharm Sin 2:1–10
Peeters AJ, Gerards W, Barendse GW, Wullems GJ (1991) In vitro flower bud formation in tobacco: interaction of hormones. Plant Physiol 97:402–408
Thanh N, Murthy H, Yu K, Hahn E, Paek K (2005) Methyl Jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-l balloon type bubble bioreactors. Appl Microbiol Biotechnol 67:197–201
Velioglu Y, Mazza G, Gao L, Oomah B (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Yan Y-H et al (2014) Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. PLoS ONE 9:e90700. doi:10.1371/journal.pone.0090700
Yang L, Stöckigt J (2010) Trends for diverse production strategies of plant medicinal alkaloids. Nat Prod Rep 27:1469–1479
Zhang J, Gao W-Y, Wang J, Li X-L (2011) Effects of explant types and media salt strength on growth and secondary metabolite accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant 33:2447–2452. doi:10.1007/s11738-011-0785-x
Zhang J, Gao W-Y, Wang J, Li X (2012) Effects of sucrose concentration and exogenous hormones on growth and periplocin accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant 34:1345–1351
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Saeed, S., Ali, H., Khan, T. et al. Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiol Mol Biol Plants 23, 229–237 (2017). https://doi.org/10.1007/s12298-016-0406-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-016-0406-7