Abstract
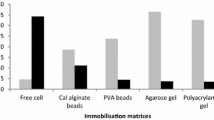
In recent years, there has been an increasing trend toward more efficient utilization of agro-industrial residues, such as sugarcane bagasse, as raw materials for industrial applications. The present study attempts to find out the effect of immobilization of Trichoderma longibrachiatum on enzyme production and immobilization of cellobiase on its kinetic activity. T. longibrachiatum was immobilized on sodium alginate (1, 1.5, and 2 % w/v) beads. The sugarcane bagasse, after autoclaving, was inoculated with 10 ml of immobilized biocatalyst and free cell culture. Maximum cellulase production was observed in immobilized fermentation system after 120 h of incubation. Cellobiase was purified up to 4.8-folds using DEAE-Sepharose column pre-equilibrated in 50 mM Tris–HCl buffer (pH 7.0) and eluted in same buffer by varying the degree of NaCl concentration. The specific activity before and after purification was 0.038 and 0.181 U/mg, respectively. The percent-entrapped activity of calcium alginate immobilized cellobiase was found maximum at 6.5 % (w/v) sodium alginate. The pH optimum of the native free enzyme was 5.5 and entrapped enzyme was 5.0. Immobilized cellobiase exhibited maximum activity at 40 °C at pH 5.5 (42.143 U). The purified enzyme was found to be highly thermostable and was found active over range of 20–80 °C and pH 4.0–8.0. The K m and V max for immobilized cellobiase from T. longibrachiatum were found 44.74 mg/ml and 79.49 mM/ml/min, respectively.



Similar content being viewed by others
References
Ahamed, A., and P. Vermette. 2008. Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochemical Engineering Journal 40: 399–407.
Ahmed, S., A. Bashir, H. Saleem, M. Saadia, and A. Jamil. 2009. Production and purification of cellulose degrading enzymes from a filamentous fungus Trichoderma harzianum. Pakistan Journal of Botany 41(3): 1411–1419.
Ahmed, S.A., N.M.A. El-Shayeb, A.M. Hashem, S.A. Saleh, and A.F. Abdel-Fattah. 2013. Biochemical studies on immobilized fungal β-glucosidase. Brazilian Journal of Chemical Engineering 30(4): 747–758.
Ashfaque, M., S. Solomon, and N. Pathak. 2014. Optimization of pretreatment and fermentation conditions for production of extracellular cellulase complex using sugarcane bagasse. Bioinformation 10(10): 606–610.
Attitalla, I.H., and B. Salleh. 2010. Improvement of carboxymethyl cellulase and xylanase production by alginate immobilized Trichoderma harzianum. Biotechnology 9: 529–532.
Bai, H., M. Irfan, Y. Wang, H. Wang, and X. Han. 2013. Immobilization of Trichoderma viride for endoglucanase and xylanase production and its application in enzymatic hydrolysis. Bothalia Journal 43(11): 82–92.
Buchert, J., A. Suurnakki, M. Tenkanen, and L. Viikari. 1996. Enzymatic characterisation of pulps. In Enzymes for pulp and paper processing, ed. Jeffries T.W., Viikari L, Vol. 655, 38–43. American Chemical Society.
Cardona, C.A., J.A. Quintero, and I.C. Paz. 2010. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresource technology 101: 4754–4766.
Chandel, A.K., S.S. Silva, W. Carvalho, and O.V. Singh. 2012. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bioproducts. Journal of Chemical Technology and Biotechnology 87: 11–20.
Dashtban, M., H. Schraft, and W. Qin. 2009. Fungal bioconversion of lignocellulosic residues; opportunities and perspectives. International Journal of Biological Science 5(6): 578–595.
Duff, S.J.B., and W.D. Murray. 1996. Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresource technology 55: 1–33.
Emregul, E., S. Sungur, and U. Akbulut. 2006. Polyacrylamide-gelatine carrier system used for invertase immobilization. Food Chemistry 97(4): 591–597.
Evstatieva, Y., M. Badalova, G. Chernev, D. Nikolova, and V. Savov. 2013. New hybrid matrix for immobilization of Trichoderma viride SL-45. Bulgarian Journal of Agricultural Science 19(2): 120–122.
Galante, Y.M., A. De Conti, and R. Monteverdi. 1998. Application of Trichoderma enzymes in food and feed industries. In Trichoderma and Gliocladium-enzymes, biological control and commercial applications, vol. 2, ed. G.F. Harman, and C.P. Kubicek, 327–342. London: Taylor & Francis.
Holker, U., M. Hofer, and J. Lenz. 2004. Biotechnological advantages of laboratory scale solid-state fermentation with fungi. Applied Microbiology and Biotechnology 64: 175–186.
Khobragade, C.N., and S.G. Chandel. 2002. Comparative study of catalytic activity of immobilized invertase in sodium alginate gel on sucrose hydrolysis. Indian Journal of Chemical Technology 9(6): 535–539.
King, A.H. 1983. Brown seaweed extracts (alginates). In Food hydrocolloids, vol. II, ed. M. Glicksman, 115–188. Boca Raton: Florida, CRC Press.
Lewis, G.E., C.W. Hunt, W.K. Sanchez, R. Treacher, G.T. Pritchard, and P. Feng. 1996. Effect of direct-fed fibrolytic enzymes on the digestive characteristics of a forage-based diet fed to beef steers. Journal of Animal Sciences 74: 3020–3028.
Mussatto, S.I., M. Fernandes, A.M.F. Milagres, and I.C. Roberto. 2008. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzyme and Microbial Technology 43: 124–129.
Onions, A.H.S., D. Allsopp, and H.O.W. Eggins. 1981. Smiths introduction to industrial mycology, 398. London: Edward Arnold.
Peinado, P.A., J.J. Moreno, J.M. Villaba, J.A. Gonzalez-Reyes, J.M. Ortega, and J.C. Mauricio. 2006. A new immobilization method and their applications. Enzyme and Microbial Technology 40: 79–84.
Rocha, G.J.M., V.F.N. Silva, C. Martinm, A.R. Goncalves, V.M. Nascimento, and A.M. SoutoMaior. 2013. Effect of xylan and lignin removal by hydrothermal pretreatment on enzymatic conversion of sugarcane bagasse cellulose for second generation ethanol production. Sugar Tech 15(4): 390–398.
Samuels, G.J., S.L. Dodd, W. Gams, L.A. Castlebury, and O. Petrini. 2002. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94: 146–170.
Shah, S., S. Nasreen, and P.A. Sheikh. 2012. Cultural and morphological characterization of Trichoderma spp. associated with green mold disease of Pleurotus spp. in Kashmir. Research Journal of Microbiology 7: 139–144.
Sukumaran, R.K., R.R. Singhania, and A. Pandey. 2005. Microbial cellulases? Production, applications and challenges. Journal of Scientific & Industrial Research 6: 832–844.
Van Elsas, J.D., A.F. Dijkstra, J.M. Govaert, and J.A. van Veen. 1986. Survival of Pseudomonas fluorescens and Bacillus subtilis introduced into two soils of different textures in field microplots. FEMS Microbiology Letters 38: 151–160.
Vijayalaxmi, S., K.A. AnuAppaiah, S.K. Jayalakshmi, V.H. Mulimani, and K. Sreeramulu. 2013. Production of bioethanol from fermented sugars of sugarcane bagasse produced by lignocellulolytic enzymes of Exiguobacterium sp. VSG-1. Applied Biochemistry and Biotechnology 171(1): 246–260.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
It is to disclose that for any aspect of the submitted work (including but not limited to grants, data monitoring board, study design, manuscript preparation, statistical analysis, etc.) has been financed from a third party (government, commercial, private foundation, etc.). There are no conflicts of interest among the authors.
Rights and permissions
About this article
Cite this article
Ashfaque, M., Solomon, S. & Pathak, N. Kinetic Study of Immobilized Cellobiase Produced from Immobilized Wild-Type Trichoderma longibrachiatum . Sugar Tech 18, 340–346 (2016). https://doi.org/10.1007/s12355-015-0410-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-015-0410-1