Abstract
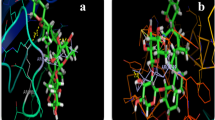
A stroke or cerebrovascular accident is a serious, life-threatening medical condition that occurs when the blood supply to part of the brain is severely reduced or cut off, depriving brain tissue of oxygen and nutrients. Studies suggested that level of gelatinases (MMP-2 and MMP-9) usually increases in the brain after stroke. The elevated activity of gelatinases plays the deleterious role in ischemic stroke, hemorrhagic stroke and perinatal hypoxic–ischemic brain injury. Therefore, matrix metalloproteinase (MMP)-2 and MMP-9 inhibition have therapeutic importance in stroke condition. Present in silico study investigates whether Withania somnifera (WS) phytochemicals inhibit the MMP-2 and MMP-9 by binding to the catalytic domain, as similar to their inhibitor or not. For that, we performed molecular docking study to evaluate the gelatinases-inhibitory potential of 36 WS phytochemicals, which compared with gelatinases inhibitors viz. hydroxamic acid, quercetin, doxycycline, minocycline and reverse hydroxamate. The results suggest that 28 out of 36 WS phytochemicals show higher affinity for MMP-2 owing to bind with active site residues of S1′-pocket with lower binding energy and smaller inhibition constant (Ki) than considered inhibitors. As well as, withanolide G and withafastuosin E show higher affinity for MMP-9 than reverse hydroxamate inhibitor. These phytochemicals have neuroprotective potential as an inherently useful oral drug to combat ischemic and hemorrhagic stroke mediated by gelatinases.




Similar content being viewed by others
References
Bramlett HM, Dietrich WD (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 24(2):133–150
Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA (2009) Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 40(4):1032–1037
Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP (2009) Hemorrhagic and ischemic strokes compared stroke severity, mortality, and risk factors. Stroke 40(6):2068–2072
Klein T, Bischoff R (2011) Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41(2):271–290
Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY (2001) MT1-MMP initiates activation of pro-MMP-2 and integrin αvβ3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res 263(2):209–223
Shapiro S, Khodalev O, Bitterman H, Auslender R, Lahat N (2010) Different activation forms of MMP-2 oppositely affect the fate of endothelial cells. Am J Physiol Cell Physiol 298(4):C942–C951
Devarajan PR, Johnston JJ, Ginsberg SS, Van Wart HE, Berliner N (1992) Structure and expression of neutrophil gelatinase cDNA. Identity with type IV collagenase from HT1080 cells. J Biol Chem 267(35):25228–25232
Ogata Y, Enghild JJ, Nagase H (1992) Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem 267(6):3581–3584
Maeda H, Okamoto T, Akaike T (1998) Human matrix metalloprotease activation by insults of bacterial infection involving proteases and free radicals. Biol Chem 379(2):193–200
Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR (1997) Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett 238(1):53–56
Rosenberg GA et al (1998) Matrix metalloproteinases and TIMPs are associated with blood–brain barrier opening after reperfusion in rat brain. Stroke 29:2189–2195
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH (2006) Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12(4):441–445
Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH (2006) Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci 26(13):3491–3495
Cunningham LA, Wetzel M, Rosenberg GA (2005) Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 50(4):329–339
Adibhatla RM, Hatcher JF (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7(3):243
Kurzepa J, Kurzepa J, Golab P, Czerska S, Bielewicz J (2014) The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci 124(10):707–716
Lakhan SE, Kirchgessner A, Tepper D, Leonard A (2013) Matrix metalloproteinases and blood–brain barrier disruption in acute ischemic stroke. Front Neurol 4:32
Jin R, Yang G, Li G (2010) Molecular insights and therapeutic targets for blood–brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis 38(3):376–385
Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC (2006) Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci 7(1):56
Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J (2003) Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol 53(6):731–742
Hernandez-Guillamon M, Martinez-Saez E, Delgado P, Domingues-Montanari S, Boada C, Penalba A, Boada M, Pagola J, Maisterra O, Rodriguez-Luna D, Molina CA (2012) MMP-2/MMP-9 plasma level and brain expression in cerebral amyloid angiopathy-associated hemorrhagic stroke. Brain Pathol 22(2):133–141
Chen W, Hartman R, Ayer R, Marcantonio S, Kamper J, Tang J, Zhang JH (2009) Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem 111(3):726–736
Crunkhorn S (2008) Stroke: widening the therapeutic window? Nat Rev Drug Discov 7(8):643
Michaelides MR, Curtin ML (1999) Recent advances in matrix metalloproteinase inhibitors research. Curr Pharm Des 5:787–820
Mishra LC (ed) (2003) Scientific basis for Ayurvedic therapies. CRC Press, Boca Raton
Raghavan A, Shah ZA (2015) Withania somnifera improves ischemic stroke outcomes by attenuating PARP1-AIF-mediated caspase-independent apoptosis. Mol Neurobiol 52(3):1093–1105
Chaudhary G, Sharma U, Jagannathan NR, Gupta YK (2003) Evaluation of Withania somnifera in a middle cerebral artery occlusion model of stroke in rats. Clin Exp Pharmacol Physiol 30(5–6):399–404
Raghavan A, Shah ZA (2015) Withania somnifera: a pre-clinical study on neuroregenerative therapy for stroke. Neural Regen Res 10(2):183
Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E, Khanna P, Jain SC, Thakur SS, Ravindranath V (2012) Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci 109(9):3510–3515
Kulkarni SK, Akula KK, Dhir A (2008) Effects of Withania sonmifera Dunal root extract against pentylenetetrazol seizure threshold in mice: possible involvement of GABAergic system. Indian J Exp Biol 46(6):465
Kataria H, Wadhwa R, Kaul SC, Kaur G (2012) Water extract from the leaves of Withania somnifera protect RA differentiated C6 and IMR-32 cells against glutamate-induced excitotoxicity. PLoS ONE 7(5):e37080
Kumar P, Kumar A (2009) Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington’s disease. J Med Food 12(3):591–600
Agnihotri AP, Sontakke SD, Thawani VR, Saoji A, Goswami VS (2013) Effects of Withania somnifera in patients of schizophrenia: a randomized, double blind, placebo controlled pilot trial study. Indian J Pharmacol 45(4):417
RajaSankar S, Manivasagam T, Sankar V, Prakash S, Muthusamy R, Krishnamurti A, Surendran S (2009) Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J Ethnopharmacol 125(3):369–373
Raghavan A (2014) Neuroprotective potential of Withania somnifera in cerebral ischemia. Doctoral dissertation, The University of Toledo
Feng Y, Likos JJ, Zhu L, Woodward H, Munie G, McDonald JJ, Stevens AM, Howard CP, De Crescenzo GA, Welsch D, Shieh HS (2002) Solution structure and backbone dynamics of the catalytic domain of matrix metalloproteinase-2 complexed with a hydroxamic acid inhibitor. Biochim Biophys Acta (BBA) Proteins Proteom 1598(1):10–23
Rowsell S, Hawtin P, Minshull CA et al (2002) Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J Mol Biol 319:173–181
Chemical Structures. National Center for Biotechnology Information. ncbi.nlm.nih.gov/pccompound. Accessed 15 Sept 2015
Search Phytochemical Databases. leffingwell.com/plants.htm. Accessed 05 Sept 2015
Kumar G, Paliwal P, Patnaik R (2016) Withania somnifera phytochemicals confer neuroprotection by inhibition of the catalytic domain of human matrix metalloproteinase-9. Lett Drug Des Discov 14:1
Gavande K, Jain K, Jain B, Mehta R (2015) Comprehensive report on phytochemistry and pharmacological prominence of Withania somnifera. UK J Pharm Biosci 3(2):15–23
Kulkarni SK, Dhir A (2008) Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry 32(5):1093–1105
Matsuda H, Murakami T, Kishi A, Yoshikawa M (2001) Structures of withanosides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withania somnifera DUNAL and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorg Med Chem 9(6):1499–1507
Kumar G, Patnaik R (2016) Exploring neuroprotective potential of Withania somnifera phytochemicals by inhibition of GluN2B-containing NMDA receptors: an in silico study. Med Hypotheses 31(92):35–43
Tursunova RN, Maslennikova VA, Abubakirov NK (1977) Withanolides in the vegetable kingdom. Chem Nat Compd 13(2):131–138
Pandey AK, Verma S, Bhattacharya P, Paul S, Mishra A, Patnaik R (2012) An in silico strategy to explore neuroprotection by quercetin in cerebral ischemia: a novel hypothesis based on inhibition of matrix metalloproteinase (MMPs) and acid sensing ion channel 1a (ASIC1a). Med Hypotheses 79(1):76–81
Liu J, Xiong W, Baca-Regen L, Nagase H, Baxter BT (2003) Mechanism of inhibition of matrix metalloproteinase-2 expression by doxycycline in human aortic smooth muscle cells. J Vasc Surg 38(6):1376–1383
Chaturvedi M, Kaczmarek L (2014) MMP-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49(1):563–573
Lee SK, Lee IH, Kim HJ, Chang GS, Chung JE, No KT (2003) The PreADME Approach: web-based program for rapid prediction of physico-chemical, drug absorption and drug-like properties. EuroQSAR 2002 Des Drugs Crop Prot Process Probl Solut 2003:418–420
Drug-likeness and molecular property prediction. Molsoft L.L.C. molsoft.com/mprop/. Accessed 27 Oct 2015
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25(2):247–260
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Cheminf 3(1):33
Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. Chem Biol Methods Protoc 1263:243–250
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17(1):57–61
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J Chem Inf Model 51(10):2778–2786
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2012) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 64:4–17
Lipinski CA (2004) Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1(4):337–341
Blake JF (2005) Identification and evaluation of molecular properties related to preclinical optimization and clinical fate. Med Chem 1(6):649–655
Leeson P (2012) Drug discovery: chemical beauty contest. Nature 481(7382):455–456
Gupta SP, Patil VM (2012) Specificity of binding with matrix metalloproteinases. In: Gupta SP (ed) Matrix metalloproteinase inhibitors. Springer, Basel, pp 35–56
Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM, Zhang C, Giritharan AB, Purnell W, Robinson CR II, Shin D (2013) Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS ONE 8(10):e76904
Hu X, Shelver WH (2003) Docking studies of matrix metalloproteinase inhibitors: zinc parameter optimization to improve the binding free energy prediction. J Mol Graph Model 22(2):115–126
Babine RE, Bender SL (1997) Molecular recognition of protein-ligand complexes: applications to drug design. Chem Rev 97(5):1359–1472
Whittaker M, Floyd CD, Brown P, Gearing AJ (1999) Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev 99(9):2735–2776
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science 295(5564):2387–2392
Jacobsen EJ, Mitchell MA, Hendges SK, Belonga KL, Skaletzky LL, Stelzer LS, Lindberg TJ, Fritzen EL, Schostarez HJ, O’Sullivan TJ, Maggiora LL (1999) Synthesis of a series of stromelysin-selective thiadiazole urea matrix metalloproteinase inhibitors. J Med Chem 42(9):1525–1536
Pandey AK, Bhattacharya P, Swet Chand Shukla SP, Patnaik R (2015) Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotection. Neural Regen Res 10(4):568
Banks WA (2009) Characteristics of compounds that cross the blood-brain barrier. BMC Neurol 9(1):1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Rights and permissions
About this article
Cite this article
Kumar, G., Patnaik, R. Inhibition of Gelatinases (MMP-2 and MMP-9) by Withania somnifera Phytochemicals Confers Neuroprotection in Stroke: An In Silico Analysis. Interdiscip Sci Comput Life Sci 10, 722–733 (2018). https://doi.org/10.1007/s12539-017-0231-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-017-0231-x