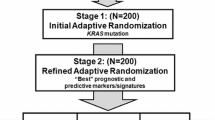
Abstract
Targeted therapies based on biomarker profiling are becoming a mainstream direction of cancer research and treatment. Depending on the expression of specific prognostic biomarkers, targeted therapies assign different cancer drugs to subgroups of patients even if they are diagnosed with the same type of cancer by traditional means, such as tumor location. For example, Herceptin is only indicated for the subgroup of patients with HER2+ breast cancer, but not other types of breast cancer. However, subgroups like HER2+ breast cancer with effective targeted therapies are rare, and most cancer drugs are still being applied to large patient populations that include many patients who might not respond or benefit. Also, the response to targeted agents in humans is usually unpredictable. To address these issues, we propose subgroup-based adaptive (SUBA), designs that simultaneously search for prognostic subgroups and allocate patients adaptively to the best subgroup-specific treatments throughout the course of the trial. The main features of SUBA include the continuous reclassification of patient subgroups based on a random partition model and the adaptive allocation of patients to the best treatment arm based on posterior predictive probabilities. We compare the SUBA design with three alternative designs including equal randomization, outcome-adaptive randomization, and a design based on a probit regression. In simulation studies, we find that SUBA compares favorably against the alternatives.






Similar content being viewed by others
References
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009
Snijders A, Nowak N, Segraves R, Blackwood S, Brown N et al (1998) Assembly of microarrays for genome-wide measurement of DNA copy number. Nat Genet 29:263–264
van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536
Curtis C, Shah S, Chin SF, Turashvili G, Rueda O, Dunning M et al (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486:346–352
Baladandayuthapani V, Ji Y, Talluri R, Nieto-Barajas L, Morris J (2010) Bayesian random segmentation models to identify shared copy number aberrations for array CGH data. J Am Stat Assoc 105: 1358–1375
Wang Z, Zang C, Rosenfeld J, Schones D, Barski A, Cuddapah S et al (2008) Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40:897–903
Barski A, Zhao K (2009) Genomic location analysis by ChIP-Seq. J Cell Biochem 107:11–18
Mitra R, Müller P, Shoudan L, Yue L, Ji Y (2013) A bayesian graphical model for chip-seq data on histone modifications. J Am Stat Assoc 108(501):69–80
Hudis CA (2007) Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med 357(1):39–51
Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G et al (2012) Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486(7404):532–536
Simon R, Maitournam A (2004) Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res 10(20):6759–6763
Sargent DJ, Conley BA, Allegra C, Collette L (2005) Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol 23(9):2020–2027
Maitournam A, Simon R (2005) On the efficiency of targeted clinical trials. Stat Med 24(3):329–339
Freidlin B, McShane LM, Korn EL (2010) Randomized clinical trials with biomarkers: design issues. J Natl Cancer Inst 102(3):152–160
Simon Richard (2010) Clinical trial designs for evaluating the medical utility of prognostic and predictive biomarkers in oncology. Pers Med 7(1):33–47
Mandrekar SJ, Sargent DJ (2010) Predictive biomarker validation in practice: lessons from real trials. Clin Trials 7(5):567–573
Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GR, Tsao A, Stewart DJ, Hicks ME, Erasmus J, Sanjay G et al (2011) The battle trial: personalizing therapy for lung cancer. Cancer Discov 1(1):44–53
Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ (2009) I-spy 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 86(1): 97–100
Yin G, Chen N, Lee JJ (2012) Phase ii trial design with bayesian adaptive randomization and predictive probability. J R Stat Soc Ser C Appl Stat 61(2):219–235
Gu X, Lee JJ (2010) A simulation study for comparing testing statistics in response-adaptive randomization. BMC Med Res Methodol 10(1):48
Zhu H, Hu F, Zhao H (2013) Adaptive clinical trial designs to detect interaction between treatment and a dichotomous biomarker. Can J Stat 41:1–15
Ruberg SJ, Chen L, Wang Y (2010) The mean does not mean as much anymore: finding sub-groups for tailored therapeutics. Clin Trials 7(5):574–583
Foster JC, Taylor JMG, Ruberg SJ (2011) Subgroup identification from randomized clinical trial data. Stat Med 30(24):2867–2880
Loredo T (2003) Bayesian adaptive exploration in a nutshell. Stat Probl Part Phys Astrophys Cosmol 1:162–165
Kruschke J (2008) Bayesian approaches to associative learning: from passive to active learning. Learn Behav 36:210–226
Fedorov VV, Leonov SL (2013) Optimal design for nonlinear response models. CRC Press
Chipman HA, George EI, McCulloch RE (1998) Bayesian cart model search. J Am Stat Assoc 93(443):935–948
Denison DGT, Mallick BK, Smith AFM (1998) A bayesian cart algorithm. Biometrika 85(2):363–377
Gelfand AE, Ghosh SK (1998) Model choice: a minimum posterior predictive loss approach. Biometrika 85(1):1–11
Raiffa H, Schlaifer R (1961) Applied statistical decision theory. Harvard Business School Publications, Boston, MA
Thall PF, Wathen JK (2007) Practical bayesian adaptive randomisation in clinical trials. Eur J Cancer 43(5):859–866
Medvedovic M, Yeung KY, Bumgarner RE (2004) Bayesian mixture model based clustering of replicated microarray data. Bioinformatics 20(8):1222–1232
Dahl DB (2006) Model-based clustering for expression data via a dirichlet process mixture model. In: Do K-A, Mueller P, Vannucci, M (eds) Bayesian inference for gene expression and proteomics, pp 201–218
Acknowledgments
The research of YJ and PM is partly supported by NIH R01 CA132897. PM was also partly supported by NIH R01CA157458. This research was supported in part by NIH through resources provided by the Computation Institute and the Biological Sciences Division of the University of Chicago and Argonne National Laboratory, under Grant S10 RR029030-01. We specifically acknowledge the assistance of Lorenzo Pesce (U of Chicago) and Yitan Zhu (NorthShore University HealthSystem).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Y., Trippa, L., Müller, P. et al. Subgroup-Based Adaptive (SUBA) Designs for Multi-arm Biomarker Trials. Stat Biosci 8, 159–180 (2016). https://doi.org/10.1007/s12561-014-9117-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12561-014-9117-1