Abstract
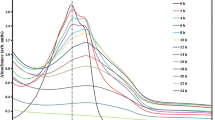
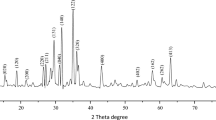
Phenol was used as a probe molecule to evaluate the photocatalytic activity of three kinds of zinc oxides, t-ZnO (tetra-needle like ZnO), n-ZnO (nano-sized particle) and c-ZnO (micro-sized particle). The photostability and effects of surface area, morphology of the photocatalyst on the photocatalytic activity were investigated in the present paper. Moreover, effects of initial concentration of the phenol and content of the photocatalysts on phenol degradation rate were systematically investigated. The results of the comparison experiments indicate that the three kinds of zinc oxides had photocatalytic activity under UV-light, and no direct relationships between the photocatalytic activity and the surface area were found for the different original ZnO particles. Instead, it is found that the particle morphology significantly affects the photocatalytic activity. The t-ZnO has a better stability and photocatalytic activity than those of n-ZnO and c-ZnO. The phenol degradation reaction follows a first-order reaction, and the degradation rate is reduced as a function of increase in the initial concentration of phenol. The optimal content of the photocatalyst is 2 g/L.
Similar content being viewed by others
References
Fujishima A., Rao T.N., and Tryk D.A., Titanium dioxide photocatalysis, Photochem. Photobiol. C: Photochem. Rev., 2000, 1(1): 1.
Garcia J.C., and Takashima K., Photocatalytic degradation of imazaquin in an aqueous suspension of titanium dioxide, Photochem. Photobiol. A: Chem., 2003, 155(1–3): 215.
Chen Shifu, and Liu Yunzhang, Study on the photocatalytic degradation of glyphosate by TiO2 photocatalyst, Chemosphere, 2007, 67(5): 1010.
Dai Ke, Peng Tianyou, and Chen Hao, Photocatalytic degradation and mineralization of commercial methamidophos in aqueous titania suspension. Environ. Sci. Technol., 2008, 42(5): 1505.
Zainal Z., Hui L.K., and Hussein M.Z., Remonel of dyes using immobilized titanium dioxide illuminated by fluorescent lamps, J.Hazard. Mater. B, 2005, 125(1): 113.
Kari Pirkanniemi and Mika Sillanpää., Heterogeneous water phase catalysis as an environmental application: a review, Chemosphere, 2002, 48(10): 1047.
Percherancier J.P., Chapelion R., and Pouyet B., Semiconductor-sensitized photodegradation of pesticides in water: the case of carbetamide, Photochem. Photobiol. A: Chem., 1995, 87(3): 261.
MCristina Yeber, Jaime Rodríguez, and Juanita Freer, Advanced oxidation of a pulp mill bleaching wastewater, Chemosphere, 1999, 39(10): 1679.
Cristian Lizama, Juanita Freer, and Jaime Baeza, Optimized photodegradation of Reactive Blue 19 on TiO2 and ZnO suspensions, Catal. Today, 2002, 76(2–4): 235.
Daneshvar N., Salari D., and Khataee A.R., Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2, Photochem. Photobiol. A: Chem., 2004, 162(2–3): 317.
Neppolian B., Sakthivel S., and Arabindoo B., Degradation of textile dye by solar light using TiO2 and ZnO photocatalysts, J. Environ. Sci. Health, Part A: Toxic/Hazardous Subst. Environ. Eng., 1999, 34(9): 1829.
Zhou Zuowan, Peng Weiming, and Ke Shaoying, Tetrapodshaped ZnO whisker and its composites, Journal of Materials Processing Technology, 1999, 89–90: 415.
Powder Diffraction File, Set 5, Joint Committee on Powder Diffraction Standards.
Khodja A.A., Sehili T., Pilichowski J.F.. and Boule P., Photocatalytic degradation of 2-phenylphenol on TiO2 and ZnO in aqueous suspensions, Journal of Photochemistry and Photobiology A: Chemistry, 2001, 141(2–3): 231.
Rosario Enríquez, and Alexander G., Agrios and pierre pichat, Probing multiple effects of TiO2 sintering temperature on photocatalytic activity in water by use of a series of organic pollutant molecules, Catal. Today, 2007, 120(2): 196.
Behnajady M.A., Modirshahla N., and Hamzavi R., Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst, Hazard. Mater., 2006, 133(1–3): 226.
Sobana N., and Swaminathan M., The effect of operational parameters on the photocatalytic degradation of acid red 18 by ZnO, Purif. Technol., 2007, 56(1): 101.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, Z., Xu, X., Duan, X. et al. Photocatalytic activity and stability of ZnO particles with different morphologies. Rare Metals 30 (Suppl 1), 183–187 (2011). https://doi.org/10.1007/s12598-011-0265-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-011-0265-x