Abstract
Purpose of Review
This review will discuss recent important trials for the treatment of HER2-positive breast cancer, emphasize ongoing areas of development, and highlight areas of unmet need.
Recent Findings
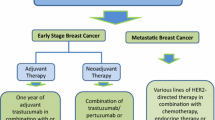
Advances for early-stage treatment have been driven by key clinical trials with pertuzumab, neratinib, and TDM-1. In the adjuvant setting, dual HER2 targeting with trastuzumab and pertuzumab has demonstrated modest improvement in disease-free survival. Neratinib also showed modest benefit in the extended adjuvant setting after prior trastuzumab. Finally, for patients who did not achieve pathologic complete response to neoadjuvant therapy, adjuvant therapy with TDM-1 showed significant disease-free survival benefit over trastuzumab. In advanced disease, neratinib appears to have activity beyond standard second line treatment. Promising compounds with early-phase data reviewed are tucatinib, margetuximab, and antibody-drug complexes. Immunotherapy in HER2-positive disease also has early-phase data. Endocrine therapy in combination with CDK4/6 inhibition and HER2-targeted therapy is in evaluation. Two areas of unmet need include CNS disease and disease treatment in the elderly population.
Summary
In the wake of recent practice changing clinical trials, HER2-directed therapy is rapidly evolving. Future advances in therapy are anticipated with ongoing studies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY). 1987;235(4785):177–82. https://doi.org/10.1126/science.3798106.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. https://doi.org/10.1056/NEJM200103153441101.
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–85. https://doi.org/10.1200/jco.2005.07.032.
Perez EA, Romond EH, Suman VJ, Jeong J-H, Davidson NE, Geyer CE, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2–positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73. https://doi.org/10.1200/JCO.2011.35.0868.
Piccart-Gebhart M, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. https://doi.org/10.1056/NEJMoa052306.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83. https://doi.org/10.1056/NEJMoa0910383.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet (London, England). 2010;375(9712):377–84. https://doi.org/10.1016/s0140-6736(09)61964-4.
Gianni L, Semiglazov V, Manikhas GM, Eiermann W, Lluch A, Tjulandin S, et al. Neoadjuvant trastuzumab in locally advanced breast cancer (NOAH): antitumour and safety analysis. J Clin Oncol. 2007;25(18_suppl):532. https://doi.org/10.1200/jco.2007.25.18_suppl.532.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5). https://doi.org/10.1093/jnci/dju055.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. https://doi.org/10.1056/NEJMoa052122.
Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):5685–92. https://doi.org/10.1200/jco.2008.21.4577.
Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34(10):1034–42. https://doi.org/10.1200/jco.2015.62.1797.
Network NCC. Breast Cancer (version 3.2019). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 4, 2019.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet (London, England). 2012;379(9816):633–40. https://doi.org/10.1016/s0140-6736(11)61847-3.
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–28. https://doi.org/10.1016/S1535-6108(04)00083-2.
•• von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–31. https://doi.org/10.1056/NEJMoa1703643. This study demonstrated DFS benefit with the addition of pertuzumab in the adjuvant setting particularly for node positive disease.
Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. https://doi.org/10.1016/S1470-2045(11)70336-9.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–84. https://doi.org/10.1093/annonc/mdt182.
• Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–77. https://doi.org/10.1016/S1470-2045(15)00551-3. This study demonstrated a modest DFS benefit with extended adjuvant therapy of neratinib particularly in high risk, hormone receptor-positive, HER2-positive disease.
Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–700. https://doi.org/10.1016/s1470-2045(17)30717-9.
Valero V, Slamon DJ, Eiermann W, Robert NJ, Pienkowski T, Martin M, et al. Efficacy results of node-negative HER2-amplified breast cancer subset from BCIRG 006 study: a phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin, and trastuzumab (TCH). J Clin Oncol. 2011;29(15_suppl):553. https://doi.org/10.1200/jco.2011.29.15_suppl.553.
Collins D, Jacob W, Cejalvo JM, Ceppi M, James I, Hasmann M, et al. Direct estrogen receptor (ER)/HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER+ breast cancer. PloS One. 2017;12(5):e0177331. https://doi.org/10.1371/journal.pone.0177331.
Hurvitz S, Chan A, Iannotti N, Ibrahim E, Chien J, Chan N, et al. Abstract P3-14-01: effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients with HER2+ early-stage breast cancer: the CONTROL trial. Cancer Res. 2018;78(4 Supplement):P3-14-01-P3-14-01. https://doi.org/10.1158/1538-7445.Sabcs17-p3-14-01.
•• von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28. https://doi.org/10.1056/NEJMoa1814017. This study demonstrated a large DFS benefit with adjuvant T-DM1 in patients with residual disease after neoadjuvant trastuzumab-based chemotherapy.
Pivot X, Romieu G, Debled M, Pierga J-Y, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741–8. https://doi.org/10.1016/S1470-2045(13)70225-0.
Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26(7):1333–40. https://doi.org/10.1093/annonc/mdv213.
• Earl HM, Hiller L, Vallier A-L, Loi S, McAdam K, Hughes-Davies L, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. The Lancet. 2019;393(10191):2599–612. https://doi.org/10.1016/S0140-6736(19)30650-6. Large randomized non-inferiority trial of 6 months adjuvant trastuzumab vs 12 months—results were positive.
• Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet (London, England). 2019;393(10191):2591–8. https://doi.org/10.1016/s0140-6736(19)30653-1. Large randomized non-inferiority trial of 6 months adjuvant trastuzumab vs 12 months—results were negative.
Carey LA, Berry DA, Ollila D, Harris L, Krop IE, Weckstein D, et al. Clinical and translational results of CALGB 40601: a neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J Clin Oncol. 2013;31(15_suppl):500. https://doi.org/10.1200/jco.2013.31.15_suppl.500.
Guarneri V, Frassoldati A, Piacentini F, Jovic G, Giovannelli S, Oliva C, et al. Preoperative chemotherapy plus lapatinib or trastuzumab or both in HER2-positive operable breast cancer (CHERLOB Trial). Clin Breast Cancer. 2008;8(2):192–4. https://doi.org/10.3816/CBC.2008.n.022.
Robidoux A, Tang G, Rastogi P, Geyer CE Jr, Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(12):1183–92. https://doi.org/10.1016/s1470-2045(13)70411-x.
Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13(2):135–44. https://doi.org/10.1016/s1470-2045(11)70397-7.
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–31. https://doi.org/10.1200/jco.2009.23.8451.
Yee D, editor. Pathological complete response predicts event-free and distant disease-free survival in the I-SPY2 TRIAL. San Antonio Breast Cancer Symposium; 2017 Dec 7, 2017; San Antonio, TX.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England). 2014;384(9938):164–72. https://doi.org/10.1016/s0140-6736(13)62422-8.
Hicks M, Macrae ER, Abdel-Rasoul M, Layman R, Friedman S, Querry J, et al. Neoadjuvant dual HER2-targeted therapy with lapatinib and trastuzumab improves pathologic complete response in patients with early stage HER2-positive breast cancer: a meta-analysis of randomized prospective clinical trials. Oncologist. 2015;20(4):337–43. https://doi.org/10.1634/theoncologist.2014-0334.
• Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2018;36(10):968–74. https://doi.org/10.1200/jco.2017.74.0126. Study showing equivalent efficacy of a biosimilar, SB3, to standard trastuzumab.
Services DoHaH. FDA-Approved Biosimilar Products. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information. Accessed October 5, 2019.
Patel TA, Ensor JE, Creamer SL, Boone T, Rodriguez AA, Niravath PA, et al. A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study). Breast Cancer Res. 2019;21(1):100. https://doi.org/10.1186/s13058-019-1186-0.
Park JW, Liu MC, Yee D, Yau C, van’t Veer LJ, Symmans WF, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11–22. https://doi.org/10.1056/NEJMoa1513750.
• Hurvitz SA, Martin M, Jung KH, Huang C-S, Harbeck N, Valero V, et al. Neoadjuvant trastuzumab (H), pertuzumab (P), and chemotherapy versus trastuzumab emtansine (T-DM1) and P in human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC): final outcome results from the phase III KRISTINE study. J Clin Oncol. 2019;37(15_suppl):500. https://doi.org/10.1200/JCO.2019.37.15_suppl.500This study showed that T-DM1 with pertuzumab resulted in inferior pCR rates compared to chemotherapy with trastuzumab and pertuzumab.
2015 Strategic Priorities Breast Cancer Steering Committee (BCSC). National Cancer Institute. 2015. https://www.cancer.gov/about-nci/organization/ccct/steering-committees/nctn/breast-cancer/bcsc-2015-strategic-priorities.pdf. Accessed September 7 2019.
Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. https://doi.org/10.1056/NEJMoa1413513.
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19. https://doi.org/10.1056/NEJMoa1113216.
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43. https://doi.org/10.1056/NEJMoa064320.
Krop IE, Kim S-B, González-Martín A, LoRusso PM, Ferrero J-M, Smitt M, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–99. https://doi.org/10.1016/S1470-2045(14)70178-0.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. https://doi.org/10.1056/NEJMoa1209124.
• Saura C OM, Feng YH, et al, editor. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. ASCO Annual Meeting; 2019. J Clin Oncol. This study demonstrated superior PFS with neratinib and capecitabine vs lapatinib and capecitabine in multiply treated metastatic HER2-positive breast cancer.
Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–9. https://doi.org/10.1200/jco.18.01511.
Hamilton E BV, Conlin A, Walker L, Moulder S, editor. Efficacy results of a phase 1b study of ONT-380, an oral HER2-specific inhibitor, in combination with capecitabine (C) and trastuzumab (T) in HER2+ metastatic breast cancer (MBC), including patients (pts) with brain metastases (mets). San Antonio Breast Cancer Symposium; 2016.
Ferrario C WS, Chaves JM, Walker LN, Krop IE, Hamilton EP, Borges VF, Moulder SL, editor. ONT-380 in the treatment of HER2+ breast cancer central nervous system (CNS) metastases (mets). ASCO Annual Meeting; 2015 2015.
Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. https://doi.org/10.1038/nature11412.
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50. https://doi.org/10.1093/annonc/mdu112.
Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2(1):56–64. https://doi.org/10.1001/jamaoncol.2015.3239.
Holgado E, Perez-Garcia J, Gion M, Cortes J. Is there a role for immunotherapy in HER2-positive breast cancer? NPJ Breast Cancer. 2018;4(1):21. https://doi.org/10.1038/s41523-018-0072-8.
• Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019;20(3):371–82. https://doi.org/10.1016/S1470-2045(18)30812-X. An important study that demonstrated activity of pembrolizumab in advanced HER2-positive disease.
Costa RLB, Soliman H, Czerniecki BJ. The clinical development of vaccines for HER2(+) breast cancer: current landscape and future perspectives. Cancer Treat Rev. 2017;61:107–15. https://doi.org/10.1016/j.ctrv.2017.10.005.
Berzofsky JA, Wood LV, Maeng H, Trepel J, Stroncek D, Morris JC. Abstract A004: HER2 cancer vaccine phase I clinical trial shows clinical benefit in 54% of evaluable patients. Cancer Immunol Res. 2019;7(2 Supplement):A004–A. https://doi.org/10.1158/2326-6074.Cricimteatiaacr18-a004.
Costa R, Zaman S, Sharpe S, Helenowski I, Shaw C, Han H, et al. A brief report of toxicity end points of HER2 vaccines for the treatment of patients with HER2(+) breast cancer. Drug Des Devel Ther. 2019;13:309–16. https://doi.org/10.2147/dddt.S188925.
Rugo HS, et al. SOPHIA primary analysis: A phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx). J Clin Oncol. 2019;37(suppl; abstr):1000.
Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–26. https://doi.org/10.1016/S1470-2045(19)30097-X.
Modi S TJ, Tamura K, Park H, Sagara Y, Murthy R, Iwata H, Krop IE, Doi T, Redfern C, Moreno-Aspitia A, Redman R, Lee C, Sugihara M, Fujisaki Y, Takahashi S, editor. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer: updated results of a large phase 1 study. San Antonio Breast Cancer Symposium; 2018 December 8, 2018
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–35. https://doi.org/10.1016/s1470-2045(19)30328-6.
Rinnerthaler G, Gampenrieder SP, Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int J Mol Sci. 2019;20(5). https://doi.org/10.3390/ijms20051115.
Fehrenbacher L CR, Geyer CE, et al, editor. NSABP B-47 (NRG Oncology): Phase III randomized trial comparing adjuvant chemotherapy with Adriamycin and cyclophosphamide followed by weekly paclitaxel, or docetaxel and cyclophosphamide with or without a year of trastuzumab in women with node-positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1+ or 2+ with negative FISH (HER2-Low IBC). San Antonio Breast Cancer Symposium; 2017 December 6, 2017.
Rimawi M, Ferrero JM, de la Haba-Rodriguez J, Poole C, De Placido S, Osborne CK, et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. J Clin Oncol. 2018;36(28):2826–35. https://doi.org/10.1200/jco.2017.76.7863.
George W, Sledge J, Toi M, Neven P, Sohn J, Inoue K, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–84. https://doi.org/10.1200/jco.2017.73.7585.
Im S-A, Lu Y-S, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–16. https://doi.org/10.1056/NEJMoa1903765.
Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–29. https://doi.org/10.1007/s10549-018-05125-4.
Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–44. https://doi.org/10.1093/annonc/mdl064.
Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5):623–8. https://doi.org/10.1016/j.breast.2014.06.009.
Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24(6):1526–33. https://doi.org/10.1093/annonc/mdt036.
Shimomura A, Tamura K, Mizutani T, Shibata T, Hara F, Fujisawa T, et al. A phase III study comparing trastuzumab emtansine with trastuzumab, pertuzumab, and docetaxel in elderly patients with advanced stage HER2-positive breast cancer: (JCOG1607 HERB TEA study). J Clin Oncol. 2019;37(15_suppl):TPS1100–TPS. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS1100.
Funding
The project described was supported in part by award number P30CA014089 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Irene Kang reports personal fees from Puma Biotechnology, and personal fees from Pfizer Inc. outside the submitted work. Janice Lu reports advisory roles for Novartis, Pfizer, Puma, Radius, and Daiichi outside the submitted work. Bing Xia reports personal fees from Genentech, personal fees from AstraZeneca, and other from Bristol-Myers Squibb outside the submitted work. Stephen Dong declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Clinical Trials
Rights and permissions
About this article
Cite this article
Kang, I., Dong, S., Lu, J. et al. Recent Developments in HER2-Directed Therapy in Breast Cancer. Curr Breast Cancer Rep 11, 311–325 (2019). https://doi.org/10.1007/s12609-019-00347-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-019-00347-x