Abstract
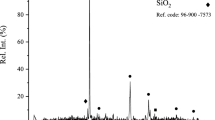
The dissolution of copper and iron from chalcopyrite concentrate in the presence of ammonium persulfate (APS) and ammonium hydroxide was investigated under atmospheric leaching conditions. Experiments were designed by central composite design (CCD). Under the optimum leaching conditions ((NH4)2S2O8 concentration = 328 g/L; NH4OH addition = 16vol%; leaching temperature = 321 K (48°C); leaching time = 120 min; liquid-to-solid ratio = 16; stirring speed = 400 r/min), selective leaching was achieved. 98.14% of the copper was leached, whereas iron did not pass into the solution. X-ray diffraction analysis of the leaching residue showed that iron compounds were predominant. Given the leaching results, the fact that the leaching process does not include uneconomical leaching stages such as extended milling/mechanical activation or high pressures/temperatures, and the low copper dissolution conditions, the attained selective leaching yield may be remarkable.
Similar content being viewed by others
References
W.G. Davenport, M.J. King, M.E. Schlesinger, and A.K. Biswas, Extractive Metallurgy of Copper, Pergamon, London, 2002.
T.S. Qiu, G.H. Nie, J.F. Wang, and L.F. Cui, Kinetic process of oxidative leaching of chalcopyrite under low oxygen pressure and low temperature, Trans. Nonferrous Met. Soc. China, 17(2007), No. 2, p. 418.
M. Al-Harahsheh, S. Kingman, and A. Al-Harahsheh, Ferric chloride leaching of chalcopyrite: Synergetic effect of CuCl2, Hydrometallurgy, 91(2008), No. 1–4, p. 89.
F. Carranza, N. Iglesias, A. Mazuelos, I. Palencia, and R. Romero, Treatment of copper concentrates containing chal-copyrite and non-ferrous sulphides by the BRISA process, Hydrometallurgy, 71(2004), No. 3–4, p. 413.
R. Padilla, M. Rodriguez, and M.C. Ruiz, Sulphidation of chalcopyrite with elemental sulphur, Metall. Mater. Trans. B, 34(2003), No. 1, p. 15.
M.D. Turan, Z.A. Sarı, and J.D. Miller, Leaching of blended copper slag in microwave oven, Trans. Nonferrous Met. Soc. China, 27(2017), No. 6, p. 1404.
M.D. Turan, M. Sarıkaya, Z.A. Sarı, A. Haxiaj, T. Depci, A. Demiraslan, and H. Nizamoğlu, Investigating and optimization of copper extraction from chalcopyrite concentrate with hydrogen peroxide in presence of acetic acid, Curr. Phys. Chem., 7(2017), No. 4, p. 267.
M.D. Turan, M. Boyrazlı, and H.S. Altundoğan, Improving of copper extraction from chalcopyrite by using NaCl, J. Cent. South Univ., 25(2018), No. 1, p. 21.
E.M. Córdoba, J.A. Muñoz, M.L. Blázquez, F. González, and A. Ballester, Leaching of chalcopyrite with ferric ion. Part III: Effect of redox potential on the silver-catalyzed process, Hydrometallurgy, 93(2008), No. 3–4, p. 97.
S. Vafaeian, M. Ahmadian, and B. Rezaei, Sulphuric acid leaching of mechanically activated copper sulphidic concentrate, Miner. Eng., 24(2011), No. 15, p. 1713.
M. Chakravorty and S. Srikanth, Kinetics of salt roasting of chalcopyrite using KCl, Thermochim. Acta, 362(2000), No. 1–2, p. 25.
R. Padilla, E. Olivares, M.C. Ruiz, and H.Y. Sohn, Kinetics of the sulfidation of chalcopyrite with gaseous sulfur, Metall. Mater. Trans. B, 34(2003), No. 1, p. 61.
K. Tkáčová and P. Baláž, Reactivity of mechanically activated chalcopyrite, Int. J. Miner. Process., 44–45(1996), p. 197.
M.S. Bafghi, A.H. Emami, A. Zakeri, and J.V. Khaki, Effect of mechanical activation on the kinetics of leaching of chal-copyrite in the ferric sulfate media, Iran. J. Mater. Sci. Eng., 7(2010), No. 2, p. 30.
A. Katsiapi, P.E. Tsakiridis, P. Oustadakis, and S. Agatzini-Leonardou, Cobalt recovery from mixed Co-Mn hydroxide precipitates by ammonia-ammonium carbonate leaching, Miner. Eng., 23(2010), No. 8, p. 643.
R.K. Nadirov, L.I. Syzdykova, A.K. Zhussupova, and M.T. Usserbaev, Recovery of value metals from copper smelter slag by ammonium chloride treatment, Int. J. Miner. Process., 124(2013), p. 145.
D. Feng and J.S.J. Van Deventer, Leaching behaviour of sulphides in ammoniacal thiosulphate systems, Hydrometallurgy, 63(2002), No. 2, p. 189.
M.D. Turan and H.S. Altundogan, Leaching of a copper flotation concentrate with ammonium persulfate in an autoclave system, Int. J. Miner. Metall. Mater., 21(2014), No. 9, p. 862.
M.D. Turan, Direct selective leaching of chalcopyrite concentrate, Can. Metall. Q., 53(2014), No. 4, p. 444.
M.D. Turan, H. Arslanoğlu, and H.S. Altundoğan, Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system, J. Taiwan Inst. Chem. Eng., 50(2015), p. 49.
G.H. Jeffery, J. Bassett, J. Mendham, and R.C. Denney, Vogel’s Textbook of Quantitative Chemical Analysis, Addison-Wesley Longman, London, 1989.
D.C. Montgomery, Design and Analysis of Experiments, John Wiley & Sons, USA, 2001.
Acknowledgement
The author would like to thank Shoeleh Assemi and her colleagues for helpful English language editing on this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turan, M.D. Optimization of selective copper extraction from chalcopyrite concentrate in presence of ammonium persulfate and ammonium hydroxide. Int J Miner Metall Mater 26, 946–952 (2019). https://doi.org/10.1007/s12613-019-1804-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1804-y