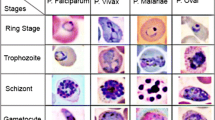
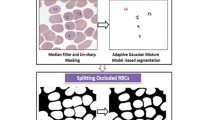
Abstract
Malaria is caused by Plasmodium parasite. It is transmitted by female Anopheles bite. Thick and thin blood smears of the patient are manually examined by an expert pathologist with the help of a microscope to diagnose the disease. Such expert pathologists may not be available in many parts of the world due to poor health facilities. Moreover, manual inspection requires full concentration of the pathologist and it is a tedious and time consuming way to detect the malaria. Therefore, development of automated systems is momentous for a quick and reliable detection of malaria. It can reduce the false negative rate and it can help in detecting the disease at early stages where it can be cured effectively. In this paper, we present a computer aided design to automatically detect malarial parasite from microscopic blood images. The proposed method uses bilateral filtering to remove the noise and enhance the image quality. Adaptive thresholding and morphological image processing algorithms are used to detect the malaria parasites inside individual cell. To measure the efficiency of the proposed algorithm, we have tested our method on a NIH Malaria dataset and also compared the results with existing similar methods. Our method achieved the detection accuracy of more than 91% outperforming the competing methods. The results show that the proposed algorithm is reliable and can be of great assistance to the pathologists and hematologists for accurate malaria parasite detection.








Similar content being viewed by others
References
Anggraini D, Nugroho AS, Pratama C, Rozi IE, Pragesjvara V, Gunawan M (2011) Automated status identification of microscopic images obtained from malaria thin blood smears using bayes decision: a study case in Plasmodium falciparum. In: 2011 International conference on advanced computer science and information systems, IEEE, pp 347–352
Bibin D, Nair MS, Punitha P (2017) Malaria parasite detection from peripheral blood smear images using deep belief networks. IEEE Access 5:9099–9108
Catanzaro B, Su B, Sundaram N, Lee Y, Murphy M, Keutzer K (2009) Efficient, high-quality image contour detection. In: Proceedings of the IEEE international conference on computer vision (ICCV), pp 2381–2388
Clendennen TE III, Long GW, Baird JK (1995) Qbc® and giemsa-stained thick blood films: diagnostic performance of laboratory technologists. Trans R Soc Trop Med Hygiene 89(2):183–184
Das DK, Ghosh M, Pal M, Maiti A, Chakraborty C (2012) Machine learning approach for automated screening of malaria parasite using light microscopic images. Micron. https://doi.org/10.1016/j.micron.2012.11.002
Das DK, Mukherjee R, Chakraborty C (2015) Computational microscopic imaging for malaria parasite detection: a systematic review. Malar J 260(1):1–19
Elter M, Haßlmeyer E, Zerfaß T (2011) Detection of malaria parasites in thick blood films. In: IEEE engineering in medicine and biology society (EMBS), pp 5140–5144, https://doi.org/10.1109/IEMBS.2011.6091273
Farid MS, Lucenteforte M, Grangetto M (2018) DOST: a distributed object segmentation tool. Multimed Tools Appl 77(16):20839–20862
Fawcett T (2006) An introduction to ROC analysis. Pattern Recognit Lett 27(8):861–874
Gatc J, Maspiyanti F, Sarwinda D, Arymurthy AM (2013) Plasmodium parasite detection on red blood cell image for the diagnosis of malaria using double thresholding. In: ICACSIS, pp 381–385, https://doi.org/10.1109/ICACSIS.2013.6761605
Hajian-Tilaki K (2013) Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp J Intern Med 4(2):627
Hung J, Goodman A, Lopes S, Rangel G, Ravel D, Costa F, Duraisingh M, Marti M, Carpenter AE (2017) Applying faster R-CNN for object detection on malaria images. CoRR arXiv:1804.09548
Jan Z, Khan A, Sajjad M, Muhammad K, Rho S, Mehmood I (2018) A review on automated diagnosis of malaria parasite in microscopic blood smears images. Multimed Tools Appl 77(8):9801–9826
Johnston SP, Pieniazek NJ, Xayavong MV, Slemenda SB, Wilkins PP, da Silva AJ (2006) Pcr as a confirmatory technique for laboratory diagnosis of malaria. Med Biol Eng Comput 44(3):1087–1089
Kaewkamnerd S, Uthaipibull C, Intarapanich A, Pannarut M, Chaotheing S, Tongsima S (2012) An automatic device for detection and classification of malaria parasite species in thick blood film. BMC Bioinform 13(17):S18
Kareem S, Kale I, Morling RC (2012) Automated malaria parasite detection in thin blood films:-a hybrid illumination and color constancy insensitive, morphological approach. In: IEEE Asia Pacific conference on circuits and systems, IEEE, pp 240–243
Le MT, Bretschneider TR, Kuss C, Preiser PR (2008) A novel semi-automatic image processing approach to determine Plasmodium falciparum parasitemia in giemsa-stained thin blood smears. BMC Cell Biol 9(1):15
Leordeanu M, Sukthankar R, Sminchisescu C (2012) Efficient closed-form solution to generalized boundary detection. In: Fitzgibbon A, Lazebnik S, Perona P, Sato Y, Schmid C (eds) Proceedings of the European conference on computer vision (ECCV), Springer, Berlin, Heidelberg, pp 516–529
Li S, Xu Y, Cong W, Ma S, Zhu M, Qi M (2018) Biologically inspired hierarchical contour detection with surround modulation and neural connection. Sensors 18(8):2559
Liang Z et al (2016) CNN-based image analysis for malaria diagnosis. In: IEEE international conference on bioinformatics and biomedicine (BIBM) pp 493–496. https://doi.org/10.1109/BIBM.2016.7822567
Linder N, Turkki R, Walliander M, Mårtensson A, Diwan V, Rahtu E, Pietikäinen M, Lundin M, Lundin J (2014) A malaria diagnostic tool based on computer vision screening and visualization of Plasmodium falciparum candidate areas in digitized blood smears. PLoS One 9(8):e104855
Mahmoud DM, Hussein HM, El Gozamy BMR, Thabet HS, Hassan MA, Meselhey RAA (2019) Screening of Plasmodium parasite in vectors and humans in three villages in Aswan Governorate. Egypt J Parasit Dis 43(1):158–163
Maiseli B, Mei J, Gao H, Yin S, Maiseli B (2014) An automatic and cost-effective parasitemia identification framework for low-end microscopy imaging devices. In: International conference on mechatronics and control (ICMC), pp 2048–2053, https://doi.org/10.1109/ICMC.2014.7231926
Malihi L, Ansari-Asl K, Behbahani A (2013) Malaria parasite detection in giemsa-stained blood cell images. In: 8th Iranian conference on machine vision and image processing (MVIP), IEEE, pp 360–365
Mohammed HA, Abdelrahman IAM (2017) Detection and classification of malaria in thin blood slide images. In: IEEE ICCCCEE, pp 1–5
Moody A (2002) Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 15(1):66–78. https://doi.org/10.1128/CMR.15.1.66-78.2002
Mushabe MC, Dendere R, Douglas TS (2013) Automated detection of malaria in giemsa-stained thin blood smears. In: IEEE engineering in medicine and biology society (EMBC), pp 3698–3701, https://doi.org/10.1109/EMBC.2013.6610346
Nasir AA, Mashor M, Mohamed Z (2012) Segmentation based approach for detection of malaria parasites using moving k-means clustering. In: IEEE-EMBS conference on biomedical engineering and sciences, IEEE, pp 653–658
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9(1):62–66. https://doi.org/10.1109/TSMC.1979.4310076
Pan WD, Dong Y, Wu D (2018) Classification of malaria-infected cells using deep convolutional neural networks. In: Machine learning-advanced techniques and emerging applications, IntechOpen
Patarakul K (2008) Role of DNA microarray in infectious diseases. Chulalongkorn Med J 52:147–153
Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G (2018) Image analysis and machine learning for detecting malaria. Transl Res In-depth Rev Diagn Med Imaging 194:36–55
Rajaraman S, Antani SK, Poostchi M, Silamut K, Hossain MA, Maude RJ, Jaeger S, Thoma GR (2018) Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images. PeerJ 6:e4568
Rosado L, Correia da Costa JM, Elias D, Cardoso SJ (2016) A review of automatic malaria parasites detection and segmentation in microscopic images. Anti-Infect Agents 14(1):11–22
Ross NE, Pritchard CJ, Rubin D, Duse A (2006) Automated image processing method for the diagnosis and classification of malaria on thin blood smears. Med Biol Eng Comput 44:427–36. https://doi.org/10.1007/s11517-006-0044-2
Savkare SS, Narote SP (2015) Automated system for malaria parasite identification. In: International conference on communication, information computing technology (ICCICT), pp 1–4, https://doi.org/10.1109/ICCICT.2015.7045660
She RC, Rawlins ML, Mohl R, Perkins SL, Hill HR, Litwin CM (2007) Comparison of immunofluorescence antibody testing and two enzyme immunoassays in the serologic diagnosis of malaria. J Travel Med 14(2):105–111
Tomasi C, Manduchi R (1998) Bilateral filtering for gray and color images. In: Proceedings of the IEEE international conference on computer vision (ICCV), IEEE Computer Society, pp 839–846
Vijayalakshmi A, Kanna BR (2019) Deep learning approach to detect malaria from microscopic images. Multimed Tools Appl. https://doi.org/10.1007/s11042-019-7162-y
Warhurst D, Williams J (1996) ACP broadsheet no 148. Laboratory diagnosis of malaria. J Clin Pathol 49(7):533
WHO (2018) World malaria report 2018. World Health Organization
WHO (2019) Global Health Observatory (GHO) data . https://www.who.int/gho/health_workforce/physicians_density/en/. Accessed June 2019
Yang D, Subramanian G, Duan J, Gao S, Bai L, Chandramohanadas R, Ai Y (2017) A portable image-based cytometer for rapid malaria detection and quantification. PLoS One 12(6):e0179161
Yang J, Price B, Cohen S, Lee H, Yang M (2016) Object contour detection with a fully convolutional encoder-decoder network. In: Proceedings of the IEEE conference on computer vision and pattern Recognition (CVPR), IEEE computer society, Los Alamitos, CA, USA
Zou L, Chen J, Zhang J, Garcia N (2010) Malaria cell counting diagnosis within large field of view. In: International conference on digital image computing: techniques and applications, pp 172–177, https://doi.org/10.1109/DICTA.2010.40
Author information
Authors and Affiliations
Contributions
TF: Conceptualization; TF and MF: methodology; TF: software; TF and MF: validation; TF and MF: writing–original draft preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The research conducted in this paper utilized the retrospective and publicly available NIH Malaria database, so that no formal consent was necessary.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatima, T., Farid, M.S. Automatic detection of Plasmodium parasites from microscopic blood images. J Parasit Dis 44, 69–78 (2020). https://doi.org/10.1007/s12639-019-01163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-019-01163-x