Abstract
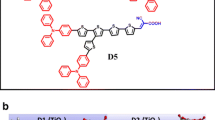
The optical, electrochemical and density functional theory molecular simulation of a metal-free D-(π-A)2, i.e., 3,3′-(5,5′-(9-hexyl-9H-carbazole-3,6-diyl)bis(thiophene-5,2-diyl))bis(2-cyanoacrylic acid) denoted as D has been investigated. A stepwise cosensitization of D with N719 dye is adopted to enhance the power conversion efficiency of dye-sensitized solar cells. The metal-free dye possesses strong absorption in the 370–450 nm wavelength range and effectively overcomes the competitive light absorption by I −3 /I −. The N719/D cosensitized dye-sensitized solar cell shows a power conversion efficiency of about 7.24 %, which is higher than the dye-sensitized solar cells based on either N719 (5.78 %) or D (3.95 %) sensitizers. The improved power conversion efficiency of the cosensitized dye-sensitized solar cell is attributed to the combined enhancement of both short-circuit photocurrent and open-circuit voltage. The short-circuit photocurrent improvement is attributed to the increase in the both light-harvesting efficiency of the cosensitized photoanode and charge collection efficiency of the dye-sensitized solar cell. However, the open-circuit voltage is improved due to better adsorption and surface coverage of TiO2 on cosensitization and an associated reduction in the back electron recombination with increased electron lifetime. These effects are analyzed using electrochemical impedance spectroscopy and dark current–voltage measurements of the dye-sensitized solar cells.







Similar content being viewed by others

References
M Gratzel Acc. Chem. Res. 42 1788 (2009)
A Hagfeldt, G Boschloo, L Sun, L Kloo and H Pettersson Chem. Rev. 110 6595 (2010)
M V Marınez-Dıaz, G de la Torre and T Torres Chem. Commun. 46 7090 (2010)
B O’Regan and M. Gratzel Nature 353 737 (1991)
A Yella et al. Science 334 629 (2011)
W Zeng et al. Chem. Mater. 22 1915 (2010)
A Mishra, M K R Fischer and P Bauerle Angew. Chem. Int. Ed. 48 2474 (2009)
A Hagfeldt, G Boschloo, L Sun, L Kloo and H Pettersson Chem. Rev. 110 6595 (2010)
Y S Yen, H H Chou, Y C Chen, C Y Hsu and J T Lin J. Mater. Chem. 22 8734 (2012)
A Abbotto et al. Dalton Trans. 40 234 (2011)
S Paek et al. Chem. Commun. 47 2874 (2011)
Y Liu et al. Chem. Commun. 47 4010 (2011)
T Maeda, Y Hamamura, K Miyanaga, N Shima, S Yagi and H Nakazumi Org. Lett. 13 5994 (2011)
S Gomez Esteban, P de la Cruz, A Aljarilla, L M Arellano and F Langa Org. Lett. 13 5362 (2011)
A Braga, S Gimenez, I Concina, A Vomiero and I N Mora-Sero J. Phys. Chem. Lett. 2 454 (2011)
C Jiao, N Zu, K-W Huang, P Wang and J Wu Org. Lett. 13 3652 (2011)
K M Lee et al. J. Power Sources 196 2416 (2011)
J Waman, F Buschest, Y Pellegtin, E Blart and F Odobel Org. Lett. 13 3944 (2011)
R Y Ogura, S Nakane, M Morooka, M Orihashi, Y Suzuki and K Noda Appl. Phys. Lett. 94 073308 (2009)
C M Lan et al. Energy Environ. Sci. 5 6460 (2012)
L Han et al. Energy Environ. Sci. 5 6057 (2012)
H Ozawa, R Shimizu and H Arakawa RSC Adv. 2 3198 (2012)
J H Yum, E Baranoff, S Wenger, M K Nazeeruddin and M Gratzel Energy Environ. Sci. 4 842 (2011)
S Mathew et al. Nature Chemistry 6 242 (2014)
J J Cid et al. Angew. Chem. Int. Ed. 46 8358 (2007)
B-W Park et al. Appl. Phys. Express 4 012301 (2011)
T Ono, T Yamaguchi and H Arakawa Sol. Energy Mater. Sol. Cells 93 831 (2009)
D Kuang et al. Langmuir 23 10906 (2007)
S-Q Fan et al. J. Phys. Chem. C 115 7747 (2011)
C-M Lan et al. Energy Environ. Sci. 5 6460 (2012)
K-M Lee et al. J. Power Sources 196 2416 (2011)
L H Nguyen et al. Phys. Chem. Chem. Phys. 14 16182 (2012)
K S V Gupta et al. Organic Electronics 15 266 (2014)
S Roquet et al. J. Am. Chem. Soc. 28 3459 (2006)
Q Wang, J E Moser and M Gratzel J. Phys. Chem. B 109 14945 (2005)
A Burke, S Ito, H Snaith, U Bach, K Kwiatkowski and M Gratzel Nano Lett. 8 977 (2008)
Z J Ning et al. J. Phys. Chem. C 113 10307 (2009)
C Klein, M K Nazeeruddin, D D Censo, P Liska and M Gratzel Inorg. Chem. 43 4216 (2004)
S Hwang et al. Chem. Commun. 2007 4887 (2007)
W Wu et al. J. Mater. Chem. 20 1772 (2010)
S Ito et al. Adv. Mater. 18 1202 (2006)
M J Frisch et al. Gaussian 03 version C01 (Wallingford CT: Gaussian, Inc) (2004)
TURBOMOLE (version 5.6) Universitat Karlsruhe (2000)
Z S Wang et al. J. Phys. Chem. B 109 3907 (2005)
M Wang et al. Nano Today 5 169 (2010)
Y Cao et al. J. Phys. Chem. C 113 6290 (2009)
J Y Kim, Y H Kim and Y S Kim Curr. Appl. Phys. 11 S117 (2011)
B J Song et al. Chem A–Eur. J. 17 11115 (2011)
S H Kang et al. J. Mater. Chem. A 1 3977 (2013)
H M Song et al. J. Mater. Chem. 22 3786 (2012)
R Kern, R Sastrawan, J Ferber, R Stangl and J Luther Electrochim. Acta 47 4213 (2002)
J Bisquert J. Phys. Chem. B 106 325 (2002)
J Bisquert Phys. Chem. Chem. Phys. 5 5360 (2003)
F Fabregat-Santiago, J Bisquert, G Garcia-Belmonte, G Boschloo and A Hagfeldt Sol. Energy Mater. Sol. Cells 87 117 (2005)
Q Wang et al. J. Phys. Chem. B 110 25210 (2006)
F Fabregat-Santiago et al. J. Am. Chem. Soc. 131 558 (2009)
J Bisquert, F Fabregat-Santiago, I Mora-Sero, G Garcia-Belmonte and S J Gimenez J. Phys. Chem. C 113 17278 (2009)
J Nissfolk, K Fredin, A Hagfeldt and G Boschloo J. Phys. Chem. B 110 17715 (2006)
Z Zhang, S M Zakeeruddin, B O’Regan, R Humphry-Baker and M Gratzel J. Phys. Chem. B 109 21818 (2005)
N Kopidakis, N R Neale and A J Frank J. Phys. Chem. B 110 12485 (2006)
M A Green Solar Cells: Operating Principles, Technology and System Applications (Englewood Cliffs, NJ: Prentice-Hall) (1982)
Acknowledgments
Manjeet Singh is grateful to Maulana Azad National Institute of Technology (MANIT), Bhopal, India, for Institute Fellowship for supporting his doctoral studies. Authors are thankful to UK India Education and Research Initiative (UKIERI-II) project coordinated by the British Council, New Delhi, India, for financial support through a Thematic Partnership. Authors are also thankful to M. Chandrasekharam, Inorganic and Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology, Hyderabad, India, for providing the metal-free dye D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, M., Kurchania, R., Pockett, A. et al. Characterization of metal-free D-(π-A)2 organic dye and its application as cosensitizer along with N719 dye for efficient dye-sensitized solar cells. Indian J Phys 89, 1041–1050 (2015). https://doi.org/10.1007/s12648-015-0681-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-015-0681-0
Keywords
- Cosensitization
- Metal-free D-(π-A)2 dye
- Dye-sensitized solar cells
- Electrochemical impedance spectroscopy