Abstract
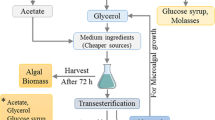
To increase the carotenoid-rich biomass production, various exogenous conditions as well as nutritional stress in terms of carbon sources were tested using a yeast strain, Rhodotorula mucilaginosa AY-01, isolated as a carotenoid producer. Biomass and carotenoid production were monitored according to the type of substrates. For cost reduction and carbon source recycling in bio-refinery, crude biomass designated to microalgal wastes was collected from Tetraselmis tertiolecta KCTC12432BP. Afterward it was used for the biodiesel production and considered as suitable carbon source for the cultivation of R. mucilaginous AY-01. The results indicated about a 27% increase in total biomass and 15.7% increase in carotenoid production respectively, as compared to glycerol supplemented medium. It is worth to note that microalgal wastes substrate can be used as an alternative carbon and nitrogen sources for the cultivation of R. mucilaginous AY-01 and contribute to the enhancement of the economic competition of biodiesel production from microalgae.





Similar content being viewed by others
References
Chisti, Y.: Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306 (2006)
Yang, F., Hanna, M.A., Sun, R.: Value-added uses for crude glycerol: a byproduct of biodiesel production. Biotechnol. Biofuels 5, 13 (2012)
de Lourdes Moreno, M., Sanchez-Porro, C., Garcia, M.T., Mellad, E.: Carotenoids’ production from halophilic bacteria. Methods Mol. Biol. 892, 207–217 (2012)
Yang, T., Sun, J., Lian, T., Wang, W., Dong, C.H.: Process optimization for extraction of carotenoids from medicinal caterpillar fungus, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms. 16, 125–135 (2014)
Tanaka, Y., Sasaki, N., Ohmiya, A.: Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 54, 733–749 (2008)
Jorgensen, K., Skibsted, L.H.: Carotenoid scavenging of radicals: effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z. Lebensm. Unters Forsch. 196, 423–429 (1993)
Woodall, A.A., Britton, G., Jackson, M.J.: Antioxidant activity of carotenoids in phosphatidylcholine vesicles: chemical and structural considerations. Biochem. Soc. Trans. 23, 133S (1995)
Yoon, K.D., Kang, S.N., Bae, J.Y., Lee, H.S., Kwak, S.S., Jang, I., Kim, I.S., Lee, C.H., Bae, J.M., Lee, S.W., Ahn, M.J.: Enhanced antioxidant and protective activities on retinal ganglion cells of carotenoids-overexpressing transgenic carrot. Curr. Drug Targets 14, 999–1005 (2013)
Frengova, G.I., Beshkova, D.M.: Carotenoids from Rhodotorula and Phaffia: yeasts of biotechnological importance. J. Ind. Microbiol. Biotechnol. 36, 163–180 (2009)
Han, M., He, Q., Zhang, W.G.: Carotenoids production in different culture conditions by Sporidiobolus pararoseus. Prep. Biochem. Biotechnol. 42, 293–303 (2012)
Bhosale, P., Gadre, R.V.: Optimization of carotenoid production from hyper-producing Rhodotorula glutinis mutant 32 by a factorial approach. Lett. Appl. Microbiol. 33, 12–16 (2001)
Aksu, Z., Tugba, E. A.: Carotenoids production by the yeast Rhodotorula mucilaginosa: use of agricultural wastes as a carbon source. Process Biochem. 40, 2985–2991 (2005)
Claudia, F., Teresa, M.P., Jose, R., Alberto, R., Teresa, L.D.S.: Selecting low-cost carbon sources for carotenoid and lipid production by the pink yeast Rhodosporidium toruloides NCYC 921 using flow cytometry. Biores. Technol. 158, 355–359 (2014)
Yoo, A.Y., Alnaeeli, M., Park, J.K.: Production control and characterization of antibacterial carotenoids from the yeast Rhodotorula mucilaginosa AY-01. Process Biochem. 51, 463–473 (2016)
Lee, C.G., Lee, J., Lee, D.G., Kim, J.W., Alnaeeli, M., Park, Y.I., Park, J.K.: Immunostimulating activity of polyhydric alcohol isolated from Taxus cuspidata. Int. J. Biol. Macromol. 85, 505–513 (2016)
Bouzidi, A., Benzarti, A., Arem, A.E., Mahfoudhi, A., Hammami, S., Gorcii, M., Mastour, M., Hellal, A.N.: Chemical composition, antioxidant and antimicrobial effects of Tunisian Limoniastrum guyonianum Durieu ex Boiss extracts. Pak. J. Pharm. Sci. 4, 1299–1305 (2016)
Yamaguchi, T., Takamura, H., Matoba, T., Terao, J.: HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 62(6), 1201–1204 (1998)
Michel, D., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Fred, S.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28(3), 350–356 (1956)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Duncombe, W.G.: The colorimetric micro-determination of long-chain fatty acids. Biochem. J. 88, 7–10 (1963)
Norton, N.: A photometric adaptation of the somogyi method for the determination of glucose. J. Biol. Chem. 1533, 75–380 (1944)
Cutzu, R., Coi, A., Rosso, F., Bardi, L., Ciani, M., Budroni, M., Zara, G., Zara, S., Mannazzu, I.: From crude glycerol to carotenoids by using a Rhodotorula glutinis mutant. World J. Microbiol. Biotechnol. 29, 1009–1017 (2013)
Taskin, M., Erdal, S.: Production of carotenoids by Rhodotorula glutinis MT-5 in submerged fermentation using the extract from waste loquat kernels as substrate. J. Sci. Food Agric. 91, 1440–1445 (2011).
Bolhassani, A.: Cancer chemoprevention by natural carotenoids as an efficient strategy. Anticancer Agents Med. Chem. 15, 1026–1031 (2015).
Bendich, A.: Carotenoids and the immune response. J. Nutr. 119, 112–115 (1989)
Acknowledgements
This research was financially supported by a grant from Marine Biotechnology Program funded by Ministry of Oceans and Fisheries, and also partially supported by grants from the Grant (GCU-2015-0057) of Gachon University, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that we have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yoo, A.Y., Amna, S. & Park, J.K. Potential Bioconversion of Microalgal Waste for Carotenoid-Rich Biomass Production. Waste Biomass Valor 9, 2053–2060 (2018). https://doi.org/10.1007/s12649-017-0009-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0009-8