Abstract
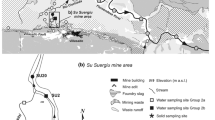
Arsenic and antimony contamination is found at the Pezinok mining site in the southwest of the Slovak Republic. Investigation of this site included sampling and analysis of water, mineralogical analyses, sequential extraction, in addition to flow and geochemical modeling. The highest dissolved arsenic concentrations correspond to mine tailings (up to 90,000 μg/L) and the arsenic is present predominately as As(V). The primary source of the arsenic is the dissolution of arsenopyrite. Concentration of antimony reaches 7,500 μg/L and its primary source is the dissolution of stibnite. Pore water in mine tailings is well-buffered by the dissolution of carbonates (pH values between 6.6 and 7.0) and arsenopyrite grains are surrounded by reaction rims composed of ferric iron minerals. Based on sequential extraction results, most solid phase arsenic is in the reducible fraction (i.e. ferric oxyhydroxides), sulfidic fraction, and residual fraction. Distribution of antimony in the solid phase is similar, but contents are lower. The principal attenuation mechanism for As(V) is adsorption to ferric oxide and hydroxides, but the adsorption seems to be limited by the competition with Sb(V) produced by the oxidation of stibnite for adsorption sites. Water in mine tailings is at equilibrium with gypsum and calcite, but far from equilibrium with any arsenic and antimony minerals. The concentrations of arsenic and antimony in the surrounding aquifer are much lower, with maximum values of 215 and 426 μg/L, respectively. Arsenic and antimony are transported by ground water flow towards the Blatina Creek, but their loading from ground water to the creek is much lower compared with the input from the mine adits. In the Blatina Creek, arsenic and antimony are attenuated by dilution and by adsorption on ferric iron minerals in stream sediments with resulting respective concentrations of 93 and 45 μg/L at the site boundary south of mine tailing ponds.







Similar content being viewed by others
References
Ahmed KM, Bhattacharya P, Hasan MA, Akhter SH, Alam SMM, Bhuyian MA, Imam MB, Khan AA, Sracek O (2004) Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Appl Geochem 19:181–200
Armienta MA, Segovia N (2008) Arsenic and fluoride in the groundwater of Mexico. Environ Geochem Health 30(4):345–353
Armienta MA, Villasenor G, Rodrigues R, Ongley LK, Mango H (1993) The role of arsenic-bearing rocks in groundwater pollution at Zimapan Valley, Mexico. Environ Geol 40:571–581
Ashley PM, Craw D, Graham BP, Chapell DA (2003) Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. J Geochem Explor 77:1–14
Bhattacharya P, Claesson M, Bundschuh J, Sracek O, Fagerberg J, Jacks G, Martin RA, del Storniolo A, Thir JM (2006) Distribution and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Sci Tot Environ 358:97–120
Blowes DW, Ptacek CJ, Jambor JL, Weisener CG (2003) The geochemistry of acid mine drainage. In: Lollar BS (ed) Environmental geochemistry, vol 9, treatise on geochemistry. Elsevier, Amsterdam, pp 149–204
Bothe JV, Brown PW (1999) The stabilities of calcium arsenates at 23 ± 1°C. J Hazard Mater 69:197–207
Cambel B (1959) Hydrothermal deposits in the Malé Karpaty Mts., mineralogy and geochemistry of their ores (in Slovak). Acta Geol Geogr Univ Comen Geol 3:1–348
Casiot C, Lebrun S, Morin G, Bruneel O, Personné JC, Elbaz-Poulichet F (2005) Sorption and redox processes controlling arsenic fate and transport in a stream impacted by acid mine drainage. Sci Tot Environ 347:122–130
Casiot C, Ujevic M, Munoz M, Seidel JL, Elbaz-Poulichet F (2007) Antimony and arsenic in a creek draining an antimony mine abandoned 85 years ago (Upper Orb basin, France). Appl Geochem 22:788–798
Chovan M, Rojkovič I, Andráš P, Hanas P (1992) Ore mineralization of the Malé Karpaty Mts. Geol Carpath 43:275–286
Chovan M, Háber M, Jelen S, Rojkovic I et al (1994) Ore textures in the Western Carpathians. Slovak Academic Press, Bratislava
Chovan M, Andráš P, Čerňanský S, DlapaP, Fľaková R, Hudáček M, Krčmář D, Kušnierová M, Lalinská B, Lux A, Majzlan J, Milovská S, Moravanský D, Ševc J, Šimoničová A, Šlesárová A, Šottník P, Uhlík P, Urík M, Ženišová Z (2006) Stanovenie rizika kontaminácie okolia Sb, Au, S ložiska Pezinok a návrh na remediáciu: toxicita As a Sb, acidifikácia. (In Slovak: Determination of the contamination risk by Sb, As, S around the Pezinok ore deposit and remediation proposal: toxicity of As and Sb, acidification), final report of the project of MŠ SR, no. AV/901/2002 (VTP25). Manuscript, Faculty of Science, Comenius University, Bratislava, p 225
DHI (2004) Mike SHE. An integrated hydrological modelling system, users guide. Danish Hydrological Institute, p 377
Donahue R, Hendry MJ (2003) Geochemistry of arsenic in uranium mine mill tailings, Saskatchewan, Canada. Appl Geochem 18:1733–1755
Egal M, Casiot C, Morin G, Elbaz-Poulichet F, Cordier MA, Bruneel O (2010) An updated insight into the natural attenuation of As concentrations in Regious Creek (southern France). Appl Geochem 25:1949–1957
ESRI (2004) What is Arc GIS, GIS by ESRI, p 76
Filella M, Belzile N, Chen Y-W (2002) Antimony in the environment: a review focused on natural waters, I. Occurrence. Earth Sci Rev 57:125–176
Flakova R, Zenisova Z, Drozdova Z, Milovská S (2005) Distribution of arsenic in surface and groundwater in Kolarsky vrch mining area (Male Karpaty Mts.) (in Slovak). Podzemna voda 11:90–103
Ford R (2005) The impact of ground-water/surface-water interactions on contaminant transport with application to an arsenic contaminated site. EPA/600/S-05/002. http://www.epa.gov/nrmrl/pubs/600s05002/epa_600_s05_002.pdf. Accessed 22 Dec 2010
Gammons CH, Nimick DA, Parker SR, Snyder DM, McCleskey RB, Amils R, Poulson SR (2008) Photoreduction fuels biogeochemical cycling of iron in Spain’s acid rivers. Chem Geol 252:202–213
Gandy CJ, Smith JWN, Jarvis AP (2007) Attenuation of mining-derived pollutants in the Hyporheic zone: a review. Sci Tot Environ 373:435–446
Giere R, Sidenko NV, Lazareva EV (2003) The role of secondary minerals in controlling the migration of arsenic and metals from high-sulphide wastes (Berikul gold mine, Siberia). Appl Geochem 18:1347–1359
Hiller E, Jurkovic L, Kordik J, Slaninka I, Jankular M, Majzlan J, Göttlicherd J, Steiningerd R (2009) Arsenic mobility from anthropogenic impoundment sediments—consequences of contamination to biota, water and sediments, Posa, Eastern Slovakia. Appl Geochem 24:2175–2185
Juillot F, Ildefonse Ph, Morin G, Calas G, de Kersabiec AM, Benedetti M (1999) Remobilization of arsenic from buried wastes at an industrial site: mineralogical and geochemical control. Appl Geochem 14:1031–1048
Jurjovec J, Ptacek CJ, Blowes DW (2002) Acid neutralization mechanisms and metal release in mine tailings: a laboratory column experiment. Geochim Cosmochim Acta 66:1511–1523
Kopřiva A, Zeman J, Sracek O (2005) High arsenic concentrations in mining waters at Kaňk, Czech Republic. In: Bundschuh J, Bhattacharya P, Chandrasekharam (eds) Natural arsenic in groundwater: occurrence, remediation and management. A.A. Balkema Publishers, Amsterdam, pp 49–56
Krupka KM, Serne RJ (2002) Geochemical factors affecting the behavior of antimony, cobalt, europium, technetium, and uranium in vadose sediments. Report PNNL-14126. Pacific Northwest National Laboratory
Langmuir D, Mahoney J, MacDonald A, Rowson J (1999) Predicting arsenic concentrations in the porewaters of buried uranium mill tailings. Geochim Cosmochim Acta 63:3379–3394
Langmuir D, Mahoney J, Rowson J (2006) Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4·2H2O) and their application to arsenic behavior in buried mine tailings. Geochim Cosmochim Acta 70:2942–2956
Lengke MF, Tempel RN (2003) Natural realgar and amorphous AsS oxidation kinetics. Geochim Cosmochim Acta 67:3281–3291
Majzlan J, Lalinská B, Chovan M, Jurkovic L, Milovska S, Göttlicher J (2007) The formation, structure, and ageing of As-rich hydrous ferric oxide at the abandoned Sb deposit Pezinok (Slovakia). Geochim Cosmochim Acta 71:4206–4220
Moravanský D, Lipka J (2004) Phyllosilicates and carbonates from hydrothermally altered metamorphic rocks in the Pezinok Sb-Au deposit, Western Carpathians, Slovakia. Miner Slovaca 36:247–264
Morin G, Calas G (2006) Arsenic in soils, mine tailings, and former industrial sites. Elements 2:97–101
Nickson RT, McArthur J, Ravenscroft P, Burgess WG, Ahmed KM (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Nordstrom DK (2002) Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountains superfund site, California. Natl Acad Sci 96:3462–4355
Parkhurst DL, Appelo CAJ (1999) PHREEQC-2, a hydrogeochemical computer program. U.S. Geological Survey Water Resources Investigation Report 99-4259
Rapant S, Vrana K, Bodiš D (1996) Geochemical atlas of Slovakia, I. part: ground water (in Slovak). Ministry of Environment of the Slovak Republic, Bratislava
Romero L, Alonso H, Campano P, Fanfani L, Cidu R, Dadea C, Keegan T, Thornton I, Farago M (2003) Arsenic enrichment in waters and sediments of the Rio Loa (Second Region, Chile). Appl Geochem 18:1399–1416
Romero FM, Armienta MA, Carillo-Chavez A (2004) Arsenic sorption by carbonate-rich aquifer material, a control on arsenic mobility at Zimapán, México, Arch. Environ Toxicol 47(1):1–13
Salzsauler KA, Sidenko NV, Sherriff BL (2005) Arsenic mobility in alteration products of sulphide-rich, arsenopyrite-bearing mine wastes, Snow Lake, Manitoba, Canada. Appl Geochem 20:2303–2314
Schreiber ME, Simo JA, Freiberg PG (2000) Stratigraphic and geochemical controls on naturally occurring arsenic in groundwater, eastern Wisconsin, USA. Hydrogeol J 8:161–176
Smedley PL, Kinniburgh DG (2002) A review of source, behaviour and distribution of arsenic in natural waters. Appl Geoch 17:517–568
Smedley PL, Kinniburgh DG, Macdonald DMJ, Nicolli HB, Barros AJ, Tullio JO, Pearce JM, Alonso (2005) Arsenic association in sediments from the loess aquifer of La Pampa, Argentina. Appl Geochem 20:989–1016
Sracek O, Bhattacharya P, Jacks G, Gustafsson JP, von Brömssen M (2004) Behavior of arsenic and geochemical modeling of arsenic enrichment in aqueous environments. Appl Geochem 19:169–180
Sracek O, Armienta MA, Rodriguez R, Villasenor G (2010) Discrimination between diffuse and point sources of arsenic in Zimapan, Hidalgo state, Mexico. J Environ Monitor 12(1):329–337
Stollenwerk KG (2003) Geochemical processes controlling transport of arsenic in groundwater: a review of adsorption. In: Welch AH, Stollenwerk KG (eds) Arsenic in ground water: geochemistry and occurrence. Kluwer, Dordrecht, pp 67–100
Trtikova S (1999) Iron ochres—products of weathering process at Fe and Sb-Au-As ore deposits of Male Karpaty (in Slovak). Dissertation, Comenius University in Bratislava
Trtikova S, Chovan M, Kusnierova M (1997) Oxidation of pyrite and arsenopyrite in the mining wastes (Pezinok-Malé Karpaty Mts.). Folia Fac Sci Nat Univ Mas Brun Geol 39:159–167
Vink BW (1996) Stability relations of antimony and arsenic compounds in the light of revised and extended Eh-pH diagrams. Chem Geol 130:12–30
Walker FP, Schreiber ME, Rimstidt JD (2006) Kinetics of arsenopyrite oxidative dissolution by oxygen. Geochim Cosmochim Acta 70:1668–1676
Whiting KS (1992) The thermodynamics and geochemistry of as with the application to subsurface waters at the Sharon steel superfund site midvale, Utah. MSc thesis, Colorado School of Mines
Williams M (2001) Arsenic in mine waters: an international study. Environ Geol 40:267–278
Wilson SC, Lockwood PV, Ashley PM, Tighe M (2010) The chemistry and behaviour of antimony in the soil environment with comparison to arsenic: a critical review. Environ Pollut 158:1169–1181
Yunmei Y, Yongsuan Z, Williams-Jones AF, Zhenmin G, Dexian L (2004) A kinetic study of the oxidation of arsenopyrite in acidic solutions: implications for the environment. Appl Geochim 19:435–444
Acknowledgments
The research was based on the results of the Slovak Research and Development Agency under the contract No. APVV-0268-06 “Contamination generated by Sb mining in Slovakia: Evaluation and strategies for remediation”. We thank an anonymous reviewer whose comments helped to improve the manuscript. We also thank Angus Calderhead for language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flakova, R., Zenisova, Z., Sracek, O. et al. The behavior of arsenic and antimony at Pezinok mining site, southwestern part of the Slovak Republic. Environ Earth Sci 66, 1043–1057 (2012). https://doi.org/10.1007/s12665-011-1310-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1310-7