Abstract
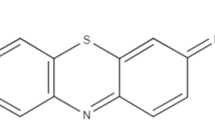
Peat of Brunei Darussalam shows a great potential for the removal of methylene blue (MB) and malachite green (MG) dyes from aqueous solution. Carefully controlled batch experiments performed by changing one parameter at a time indicate that the optimum time periods of agitation and settling required for maximum removal of MB are 2.0 and 1.0 h, respectively, while these values for MG are 4.0 and 1.0 h, respectively. The optimum pH is determined to be the ambient value, and under the optimum conditions, 90 % removal of both dyes was determined under laboratory conditions. The equilibrium adsorption data analyzed for various isotherm models suggest that the Sips and Redlich–Peterson (R–P) models are valid for MB and MG, respectively. Further, thermodynamic studies show that the adsorption of both dyes on peat is spontaneous and endothermic. The adsorption capacities (q max) of MB and MG dyes on peat are 0.45 and 0.31 mmol g−1, respectively. Characterization of the surfaces of peat before and after treatment of dyes by SEM and FTIR provides conclusive evidence of adsorption of both dyes. Kinetics studies indicate that the adsorption of both MB and MG dyes is favored toward the pseudo-second-order model, with a little contribution of MG to the pseudo-first-order model. These results suggest that peat is a potential low-cost adsorbent for the removal of MB and MG dyes.










Similar content being viewed by others
References
Altinisik A, Gur E, Seki Y (2010) A natural sorbent, Luffa cylindrica for the removal of a model basic dye. J Hazard Mater 179:658–664
Aravindhan R, Rao JR, Nair BU (2007) Removal of basic yellow dye from aqueous solution by sorption on green alga Caulerpa scalpelliformis. J Hazard Mater 142:68–76
Baek M, Ijagbemi CO, Se-Jin O, Kim D (2010) Removal of malachite green from aqueous solution using degreased coffee bean. J Hazard Mater 176:820–828
Barka N, Ouzaouit K, Abdennouri M, Makhfouk ME (2013) Dried prickly pear cactus (Opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J Taiwan Inst Chem Eng 44:52–60
Bjornbom E, Bjornbom P, Sjostrom K (1991) Energy-rich components and low-energy components in peat. Fuel 70:177–180
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solution by organic zeolites. II. Kinetics. J Am Chem Soc 59:2836–2848
Brown PA, Gill SA, Allen SJ (2000) Metal removal from wastewater using peat. Water Res 16:3907–3916
Buvaneswari N, Kannan C (2011) Plant toxic and non-toxic nature of organic dyes through adsorption mechanism on cellulose surface. J Hazard Mater 189:294–300
Cardoso NF, Lima EC, Pinto IS, Amavisca CV, Royer B, Pinto RB, Alencar WS, Pereira SFP (2011) Application of cupuassu shell as biosorbent for the removal of textile dyes from aqueous solution. J Environ Manage 92:1237–1247
Cengiz S, Cavas L (2008) Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Bioresour Technol 99:2357–2363
Chathuranga PKD, Priyantha N, Iqbal SS, Iqbal MCM (2013) Biosorption of Cr(III) and Cr(VI) species from aqueous solution by Cabomba caroliniana: kinetic and equilibrium study. Environ Earth Sci 70:661–671
Chieng HI, Lim LBL, Priyantha N, Tennakoon DTB (2013) Sorption characteristics of peat of Brunei Darussalam III: equilibrium and kinetics studies on adsorption of crystal violet (CV). Int J Earth Sci Eng 6:791–801
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Dahri MK, Kooh MRR, Lim LBL (2013) Removal of methyl violet 2B from aqueous solution using Casuarina equisetifolia needle. ISRN Environ Chem. doi:10.1155/2013/619819
Din ATM, Hameed BH (2010) Adsorption of methyl violet dye on acid modified activated carbon: isotherms and thermodynamics. J Appl Sci Environ Sanit 5:161–170
Etci Ö, Bektaş N, Öncel MS (2010) Single and binary adsorption of lead and cadmium ions from aqueous solution using the clay mineral beidellite. Environ Earth Sci 61:231–240
Faouzi EM, Bensalah N, Gadri A (2009) Treatment of aqueous wastes contaminated with Congo Red dye by electrochemical oxidation and ozonation processes. J Hazard Mater 168:1163–1169
Fernandes AN, Almeida CAP, Menezes CTB, Debacher NA, Sierra MMD (2007) Removal of methylene blue from aqueous solution by peat. J Hazard Mater 144:412–419
Freundlich HMF (1906) Over the adsorption in the solution. J Phys Chem 57:385–470
Gandois L, Cobb AR, Chieng HI, Lim LBL, Salim KA, Harvey CF (2012) Impact of deforestation on solid and dissolved organic matter characteristics of tropical peat forests: implications for carbon release. Biogeochemistry 114:183–199
Ghaedi M, Taghavimoghadam N, Naderi S, Sahraei R, Daneshfar A (2013) Comparison of removal of bromothymol blue from aqueous solution by multiwalled carbon nanotube and Zn(OH)2 nanoparticles loaded on activated carbon: a thermodynamic study. J Ind Eng Chem 19:1493–1500
Graham D (1955) Characterization of physical adsorption systems. III. The separate effects of pore size and surface acidity upon the adsorbent capacities of activated carbons. J Phys Chem 59:896–900
Gupta N, Kushwaha AK, Chattopadhyaya MC (2011) Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab J Chem. doi:10.1016/j.arabjc.2011.07.021
Hamdaoui O (2006) Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J Hazard Mater 135:264–273
Hamdaoui O, Saoudi F, Chiha M, Naffrechoux E (2008) Sorption of malachite green by a novel sorbent, dead leaves of plane tree: equilibrium and kinetic modelling. Chem Eng J 143:73–84
Hameed BH (2009) Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J Hazard Mater 162:939–944
Hameed BH, Ahmad AA (2009) Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J Hazard Mater 166:233–238
Hameed BH, El-Khaiary MI (2008) Batch removal of malachite green from aqueous solutions by adsorption on oil palm trunk fibre: equilibrium isotherms and kinetic studies. J Hazard Mater 154:237–244
Hamidpour M, Kalbasi M, Afyuni M, Shariatmadari H, Furrer G (2011) Sorption of lead on Iranian bentonite and zeolite: kinetics and isotherms. Environ Earth Sci 62:559–568
Han R, Wang Y, Han P, Shi J, Yang J, Lu Y (2006) Removal of methylene blue from aqueous solution by chaff in batch mode. J Hazard Mater 137:550–557
Han RP, Zhang LJ, Song C, Zhang MM, Zhu HM, Zhu LJ (2010) Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr Polym 79:1140–1149
Han X, Wang W, Ma X (2011) Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf. Chem Eng J 171:1–8
Hararah MA, Al-Nasir F, El-Hasan T, Al-Muhtaseb AH (2012) Zinc adsorption–desorption isotherms: possible effects on the calcareous vertisol soils from Jordan. Environ Earth Sci. doi:10.1007/s12665-011-1188-4
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Huat BBK, Kazemian S, Prasad A, Barghchi M (2011) State of an art review of peat: general perspective. Int J Phys Sci 6:1988–1996
Kumar KV (2006) Comparative analysis of linear and non-linear method of estimating the sorption isotherm parameters for malachite green onto activated carbon. J Hazard Mater 136:197–202
Lagergren S (1898) Zur theorie der sogenannten adsorption gelőster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 24:1–39
Langmuir I (1917) The constitution and fundamental properties of solids and liquids. II. Liquids. 1. J Am Chem Soc 39:1848–1906
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lim LBL, Priyantha N, Tennakoon DTB, Dahri MK (2012) Biosorption of cadmium(II) and copper(II) ions from aqueous solution by core of Artocarpus odoratissimus. Environ Sci Pollut Res 19:3250–3256
Lim LBL, Priyantha N, Chieng HI, Muhd Dahri K, Tennakoon DTB, Zehra T, Suklueng M (2013a) Artocarpus odoratissimus skin as a potential low-cost biosorbent for the removal of methylene blue and methyl violet 2B. Desalination Water Treat. doi:10.1080/19443994.2013.852136:1-12
Lim LBL, Priyantha N, Tennakoon D, Zehra T (2013b) Sorption characteristics of peat of Brunei Darussalam. II: interaction of aqueous copper(II) species with raw and processed peat. J Ecotechn Res 17:45–49
Lim LBL, Priyantha N, Tennakoon DTB, Chieng HI, Bandara C (2013c) Sorption characteristics of peat of Brunei Darussalam I: characterization of peat and adsorption equilibrium studies of methylene blue—peat interactions. Ceylon J Sci 17:41–51
Mall ID, Srivastava VC, Agarwal NK, Mishra IM (2005) Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids Surf A Physicochem Eng Asp 264:17–28
Mckay G, Ho YS (1999) Pseudo-second-order model for sorption process. Proc Biochem 34:451–465
Mui ELK, Cheung WH, Valix M, McKay G (2010) Dye adsorption onto char from bamboo. J Hazard Mater 177:1001–1005
Nethaji S, Sivasamy A, Thennarasu G, Saravanan S (2010) Adsorption of malachite green dye onto activated carbon derived from Borassus aethiopum flower biomass. J Hazard Mater 181:271–280
Nuithitikul K, Srikhun S, Hirunpraditkoon S (2010) Kinetics and equilibrium adsorption of basic green 4 dye on activated carbon derived from durian peel: effects of pyrolysis and post-treatment conditions. J Taiwan Inst Chem Eng 41:591–598
Oliveira L, Franca A, Alves T, Rocha S (2008) Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J Hazard Mater 155:507–512
Oren MJ, MacKay GDM (1986) Rheological and calorific properties of peat-in-oil slurries. Fuel 65:644–646
Ozer A, Dursun G (2007) Removal of methylene blue from aqueous solution by dehydrated wheat bran carbon. J Hazard Mater 146:262–269
Poots VJP, McKay G (1979) The specific surfaces of peat and wood. J Appl Polym Sci 23:1117–1129
Rani RD, Sasidhar P (2012) Geochemical and thermodynamic aspects of sorption of strontium on kaolinite dominated clay samples at Kalpakkam. Environ Earth Sci 65:1265–1274
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024–1029
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Santhi T, Manonmani S, Vasantha VS, Chang YT (2011) A new alternative adsorbent for the removal of cationic dyes from aqueous solution. Arab J Chem. doi:10.1016/j.arabjc.2011.06.004
Shirmardi M, Mahvi AH, Hashemzadeh B, Naeimabadi A, Hassani G, Niri MV (2013) The adsorption of malachite green (MG) as a cationic dye onto functionalized multi walled carbon nanotubes. Korean J Chem Eng 30:1603–1608
Sips R (1948) Combined form of Langmuir and Freundlich equations. J Chem Phys 16:490–495
Tahir SS, Rauf N (2006) Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842–1848
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154:337–346
Uddin MT, Islam MA, Mahmud S, Rukanuzzaman M (2009) Adsorptive removal of methylene blue by tea waste. J Hazard Mater 164:53–60
Unuabonah EI, Adie GU, Onah LO, Adeyemi OG (2009) Multistage optimization of the adsorption of methylene blue dye onto defatted Carica papaya seeds. Chem Eng J 155:567–579
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng 89:31–60
Wust RAJ, Ward CR, Bustin RM, Hawke MI (2002) Characterization and quantification of inorganic constituents of tropical peats and organic-rich deposits from Tasek Bera (Peninsular Malaysia): implications for coals. Int J Coal Geol 49:215–249
Yagub MT, Sen TK, Ang M (2013) Removal of cationic dye methylene blue (MB) from aqueous solution by ground raw and base modified pine cone powder. Environ Earth Sci. doi:10.1007/s12665-013-2555-0
Yasemin B, Haluk A (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194:259–267
Zengin G (2013) Effective removal of zinc from an aqueous solution using Turkish leonardite–clinoptilolite mixture as a sorbent. Environ Earth Sci. doi:10.1007/s12665-013-2364-5
Acknowledgments
The authors would like to thank the Government of Brunei Darussalam and the Universiti Brunei Darussalam (UBD) for their financial support. The authors would also like to thank the Biology and Energy Programmes at UBD for the use of SEM and XRF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chieng, H.I., Zehra, T., Lim, L.B.L. et al. Sorption characteristics of peat of Brunei Darussalam IV: equilibrium, thermodynamics and kinetics of adsorption of methylene blue and malachite green dyes from aqueous solution. Environ Earth Sci 72, 2263–2277 (2014). https://doi.org/10.1007/s12665-014-3135-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3135-7