Abstract
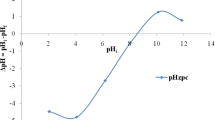
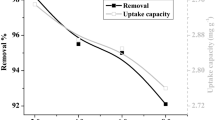
The present study highlights the use of forest soil for glyphosate removal from aqueous solutions under different experimental conditions through batch mode and optimizing the process conditions. Maximum glyphosate adsorption (161.29 mg/g) occurred at pH 12. Experimental data of glyphosate biosorption onto forest soil fitted well to Langmuir isotherm model in comparison with the Freundlich and D–R model. Kinetic studies were carried out to get useful information on the rate of glyphosate adsorption onto the forest soil, which was found to follow pseudo-second-order model. Four important process parameters including glyphosate concentration (5–30 mg/L), pH (2–14), contact time (5–120 min) and dose (0.05–1.5) were optimized to obtain the best response of glyphosate removal using the statistical Box–Behnken design. Optimized values of initial glyphosate concentration, pH, contact time and dose were found as 25.74 mg/L, 12.0, 119.99 min and 0.05 g, respectively, with the 87.8% removal efficiency. The efficiency of the operating variables was also assessed by the percent contribution and Pareto analysis. Thermodynamics data suggest that glyphosate adsorption is spontaneous in nature. The results revealed that forest soil can be used as an effective and low-cost adsorbent to remove glyphosate from aqueous solutions.













Similar content being viewed by others
References
Akhtar M, Hasany SM, Bhanger MI, Iqbal S (2007) Low cost sorbents for the removal of methyl parathion pesticide from aqueous solution. Chemosphere 66:1829–1838
Aksu Z, Kabasakal E (2004) Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep Purif Technol 35(3):223–240
Al-Qodah Z, Shawaqfeh T, Lafi WK (2007) Adsorption of pesticides from aqueous solutions using oil shale ash. Desalination 208(1–3):294–305
Aleboyeh A, Daneshvar N, Kasiri MB (2008) Optimization of C.I. Acid red 14 azo dye removal by electrocoagulation batch process with response surface methodology. Chem Eng Process 47:827–832
Amin MN, Mustafa AI, Khalil MI, Rahman M, Nahid I (2012) Adsorption of phenol onto rice straw biowaste for water purification. Clean Technol Environ Policy 14:837–844. doi:10.1007/s10098-012-0449-6
APHA (2001) Revisions to standard methods for the examination of water and wastewater (supplement). American Public Health Association, Washington DC
Ayranci E, Hoda N (2004) Adsorption of bentazon and propanil from aqueous solutions at the high area activated carbon-cloth. Chemosphere 57:755–762
Ayranci E, Hoda N (2005) Adsorption kinetics and isotherms of pesticides onto activated carbon-cloth. Chemosphere 60:1600–1607
Benetoli LdeB, Santana Hde, Zaia CEACeDAM (2010) Adsorption of glyphosate in a forest soil: a study using Mössbauer and FT-IR spectroscopy. Quim Nova 33:855–859
Bhaskara BL, Nagaraja P (2006) Direct sensitive spectrophotometric determination of glyphosate by using ninhydrin as a chromogenic reagent in formulations and environmental water samples. Helv Chim Acta 89:2686–2693
Bhaumik R, Mondal NK (2015) Adsorption of fluoride from aqueous solution by a new low-cost adsorbent: thermally and chemically activated coconut fibre dust. Clean Tech Environ Policy. doi:10.1007/s10098-015-0937-6
Carneiro RT, Taketa TB, Gomes NRJ, Oliveira JL, Campos EV, de Moraes MA, da Silva CM, Beppu MM, Fraceto LF (2015) Removal of glyphosate herbicide from water using biopolymer membranes. J Environ Manag 151:353–360. doi:10.1016/j.jenvman.2015.01.005
Chattoraj S, Sadhukhan B, Mondal NK (2013) Predictability by Box–Behnken model for carbaryl adsorption by soils of Indian origin. J Environ Sci Health Part B 48:626–636
Chattoraj S, Mondal NK, Das B, Roy P, Sadhukhan B (2014) Carbaryl removal from aqueous solution by Lemna major biomass using response surface methodology and artificial neural network. J Environ Chem Eng 2:1920–1928
Chattoraj S, Mondal NK, Sadhukhan B, Roy P, Roy TK (2016) Optimization of adsorption parameters for removal of carbaryl insecticide using neem bark dust by response surface methodology. Water Conserv Sci Eng 1:127–141
Cheah VB, Kirkwood RC, Lum KY (1997) Adsorption, desorption and mobility of four commonly used pesticides in Malaysian agricultural soils. Pestic Sci 50:53–63
Daneshvar N, Aber S, Seyed Dorraji MS, Khataee AR, Rasoulifard MH (2007) Photocatalytic degradation of the insecticide diazinon in the presence of prepared nanocrystalline ZnO powders under irradiation of UV–C light. Sep Purif Technol 58:91–98
Derylo-Marczewska A, Blachnio M, Marczewaski AW, Swiatkowski A, Tarasink B (2010) Adsorption of selected herbicides from aqueous solutions on activated carbon. J Therm Anal Calorim 101:785–794
Freundlich HM (1907) On adsorption in solution. J Phys Chem 57:385–471
Ghosh SB, Bhaumik R, Mondal NK (2016) Optimization study of adsorption parameters for removal of fluoride using aluminium-impregnated potato plant ash by response surface methodology. Clean Technol Environ Policy 18:1069–1083. doi:10.1007/s10098-016-1097-z
Gupta VK, Ali I (2008) Removal of endosulfan and methoxychlor from water on carbon slurry. Environ Sci Technol 42(3):766–770
Gupta VK, Ali I, Suhas Saini VK (2006) Adsorption of 2,4-D and carbofuran pesticides using fertilizer and steel industry wastes. J Colloid Interface Sci 299:556–563
Hamadi NK, Swaminathan S, Chen XD (2004) Adsorption of paraquat dichloride from aqueous solution by activated carbon derived from used tires. J Hazard Mate B 122:133–141
Hameed BH, Salman JM, Ahmad AL (2009) Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J Hazard Mater 16(1):121–126
Herath I, Vithanage M, Kumarathilaka P, Bandara T, Jayawardhana Y, Mayakaduwa S, Wickramasinghe S (2015) Rice husk derived engineered biochar for glyphosate removal in aqueous media. J Environ Indic 9:41
Hu YS, Zhao YQ, Sorohan B (2011) Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination 271(1–3):150–156
Jain M, Garg VK, Kadirvelu K (2009) Chromium(VI) removal from aqueous system using Helianthus annus (sunflower) stem waste. J Hazards Mater 162:365–372
Kesraoui-Abdessalem A, Oturan N, Bellakhal N, Dachraoui M, Oturan MA (2008) Experimental design methodology applied to electroFenton treatment for degradation of herbicide chlortoluron. Appl Catal B Environ 78:334–341
Kogan M, Metz A, Ortega R (2003) Adsorption of glyphosate in Chilean sols and its relationship with unoccupied phosphate binding sites. Pesq Agropec Bras 38(4):513–519
Kogel-knabner I, Zeeh W, Hatcher PG (1988) Chemical composition of the organic matter in forest soils: the humas layer. J Plant Nutr Soil Sci 151:331–340
Korbahti BK, Rauf MA (2008) Application of response surface analysis to the photolytic degradation of basic red 2 dye. Chem Eng J 138:166–171
Krapac IG, Roy WR, Smyth CA, Barnhardt ML (2008) Occurrence and distribution of pesticides in soil at agrichemical facilities in Illinois. J Soil Cont 4(3):209–226
Kubilay S et al (2007) Removal of Cu(II), Zn(II), and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 13(1):41–51
Mayakaduwa SS, Kumarathilaka P, Herath I, Ahmad M, Al-Wabel M, Ok YS, Usman A, Abduljabbar A, Vithanage M (2016) Equilibrium and kinetic mechanisms of woody biochar on aqueous glyphosate removal. Chemosphere 144:2516–2521. doi: 10.1016/j.chemosphere.2015.07.080
Memon GZ, Bhanger MI, Akhtar M, Talpur FN, Memon JR (2008) Adsorption of methyl parathion pesticide from water using watermelon peels as a low cost adsorbent. Chem Eng J 138:616–621
Miles C, Moye H (1988) Extraction of glyphosate herbicide from soil and clay minerals and determination of residues in soils. J Agric Food Chem 36:486–491
Mondal MK (2010) Removal of Pb(II) from aqueous solution by adsorption using activated tea waste. Korean J Chem Eng 27:144–151. doi:10.1007/s11814-009-0304-6
Mondal NK, Chattoraj S, Sadhukhan B, Das B (2013) Evaluation of carbaryl sorption in alluvial soil. Songklanakarin J Sci Technol 35:727–738
Piccolo A, Celano G, Agrienzo M, Mirabella A (1994) Adsorption and desorption of glyphosate in some Europeans soil. J Environ soil Health 6:1105–1115
Piccolo A, Celano G, Conte P (1996) Adsorption of glyphosate by humic substances. J Agric Food Chem 44:2442–2446
Prata F, Lavorenti A, Regitano JB, Tornisielo VL (2000) Influência da matéria orgânica na sorção e dessorção do glifosato em solos com diferentes atributos mineralógicos. Revista Brasileira de Ciência do Solo 24:947–951
Prata F, do Brasil Cardinalia VC, Lavorenti A, Tomisielo VL, Regiteno JB (2003) Glyphosate sorption and desorption in soils with distinct phosphorus levels. Sci Agric 60(1):175–180
Putra et al (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from waste water: mechanisms, isotherms and kinetics. Water Res 43(9):2419–2430
Pyrzynska K, Stafiej A, Biesaga M (2007) Sorption behavior of acidic herbicides on carbon nanotubes. Microchim Acta 159:293–298
Rahmanifar B, Dehaghi SM (2014) Removal of organochlorine pesticides by chitosan loaded with silver oxide nanoparticles from water. Clean Technnol Environ Policy 16:1781–1786. doi:10.1007/s10098-013-0692-5
Reddy NA, Lakshmipathy R, Sarada NC (2014) Application of Citrullus lanatus rind as biosorbent for removal of trivalent chromium from aqueous solution. Alex Eng J 53:969–975
Rhoades JD (1982) Cation exchange capacity. In: Page AL (ed) Methods of soil analysis. Part 2: Chemical and microbiological properties, 2nd edn. Agronomy 9:149–157
Roberts TR, Hutson DH, Lee PW, Nicholls PH, Plimmer JR (1998) Metabolic pathways of agrochemicals. Part 1: herbicides and plant growth regulators. The Royal Society of Chemistry, pp 386–400
Saha P, Chowdhury S, Gupta S, Kumar I (2010) Insight into adsorption equilibrium, kinetics and thermodynamics of malachite green onto clayey soil of Indian origin. Chem Eng J 167:874–882
Salman JM, Abid FM, Muhammed AA (2012) Batch study for pesticide glyphosate adsorption onto palm oil fronds activated carbon. Asian J Chem 24(12):5646–5648
Sathishkumar M, Choi JG, Ku CS, Vijayaraghavan K, Binupriya AR, Yun SE (2009) Carbaryl sorption by porogen-treated banana pith carbon. Adsorpt Sci Technol 26:679–686
Singh KP, Rai P, Pandey P, Sinha S (2012) Modeling and optimization of trihalomethanes formation potential of surface water (a drinking water source) using Box-Behnken design. Environ Sci Pollut Res 19:113–127
Srividya K, Mohanty K (2009) Biosorption of hexavalent chromium from aqueous solutions by Catla catla scales: equilibrium and kinetics studies. Chem Eng J 155:666–673
Tevez HR, Afonso MS (2015) pH dependence of glyphosate adsorption on soil horizons. Boletin De La Sociedad Mexicana 67(3):509–516
Weber WJ, Morris JC (1963) Intraparticle diffusion during the sorption of surfactants onto activated carbon. J Sanit Eng Div Am Soc Civ Eng 89(1):53–61
Zaroual Z, Chaair H, Essadki AH, EI Ass K, Azzi M (2009) Optimizing the removal of trivalent chromium by electrocoagulation using experimental design. Chem Eng J 148:488–495
Zhao YQ, Razali M, Babatunde AO, Yang Y, Bruen M (2007) Reuse of aluminium-based water treatment sludge to immobilize a wide range of phosphorus contamination: equilibrium study with different isotherm models. Sep Sci Technol 42(12):2705–2721
Zhou T, Lim TT, Chin SS, Fane AG (2011) Treatment of organics in reverse osmosis concentrate from a municipal wastewater reclamation plant: feasibility test of advanced oxidation processes with/without pretreatment. Chem Eng J 166:932–939
Zodi S, Potier O, Lapicque F, Leclerc JP (2010) Treatment of industrial waste waters by electrocoagulation: optimization of coupled electrochemical and sedimentation processes. Desalination 261:186–190
Acknowledgements
Authors acknowledge their sincere thanks to all faculty members of the Department of Environmental Science, The University of Burdwan, Burdwan, West Bengal, for their unconditional support to execute the present research work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sen, K., Mondal, N.K., Chattoraj, S. et al. Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology. Environ Earth Sci 76, 22 (2017). https://doi.org/10.1007/s12665-016-6333-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6333-7