Abstract
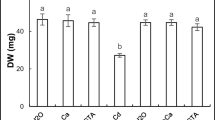
The effect of calcium (Ca) on lentil (Lens culinaris Medic.) seedlings exposed to cadmium (Cd) stress was studied by investigating plant growth and antioxidant enzyme activities. Plants were grown for 14 days in full-strength Hoagland nutrient media supplemented with Cd concentrations of 0, 10, 20, and 40 μM, and on corresponding medium supplied with 5 mM Ca(NO3)2 prior to Cd addition. Increasing Cd led to accumulation of metal and reduced the fresh weight of the shoots more strongly than that of the roots. Cd concentrations of 20 and 40 μM were selected to study its toxic effect on seedlings. Activities of superoxide dismutase, ascorbate peroxidase, catalase, dehydroascorbate reductase, and glutathione reductase decreased at much higher magnitude in the shoots than those observed in the roots under Cd exposure. Failure of antioxidant defense in scavenging of reactive oxygen species was evidenced by abnormal rise in H2O2, resulting in enhancement of lipid peroxidation and membrane electrolyte leakage as the marks of Cd-induced oxidative stress in lentil seedlings. Ca priming in the media significantly reduced the Cd accumulation and considerably alleviated the adverse impact of Cd treatment by modulating the antioxidant enzyme activity. Mitigation of Cd-induced stress by Ca application was strongly suggested by declining levels of H2O2 and consequent lowering of oxidative damage of membrane. Consequently, this enhanced fresh mass of plant parts as the sign of Ca-mediated normal growth in Cd-treated lentil seedlings.
Similar content being viewed by others
References
Akibode S, Maredia M. 2011. Global and regional trends in production, trade and consumption of food legume crops. Draft Report, March 27, 2011
Anjum NA, Umar S, Iqbal M, Khan NA. 2011. Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate-glutathione cycle metabolism. Russ. J. Plant Physiol. 58: 92–99
Bandeolu E, Eyidogan F, Yücel M, Öktem HA. 2004. Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul. 42: 69–77
Beyer WF, Fridovich I. 1987. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 161: 559–566
Bradford MM. 1976. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Carlberg I, Mannervik B. 1985. Glutathione reductase. In M Alton, eds, Methods in Enzymology, Academic Press, San Diego, pp 484–490
Chance B, Maehly AC. 1955. Assay of catalases and peroxidases. Methods Enzymol. 2: 764–817
Chen YL, Huang RF, Xiao YM, Lu P, Chen J, Wang XC. 2004. Extracellular calmodulin-induced stomatal closure is mediated by heterotrimeric G protein and H2O2. Plant Physiol. 136: 4096–4103
Choi YE, Harada E, Wada M, Tsuboi H, Morita Y, Kusano T, Sano H. 2001. Detoxification of cadmium in tobacco plants: Formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 213: 45–50
Clemens S. 2006. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88: 1707–1719
DalCorso G, Farinati S, Furini A. 2010. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 5: 663–667
De Pinto MC, De Gara L. 2004. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J. Exp. Bot. 55: 2559–2569
Dionisio-Sese M, Tobita S. 1998. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135: 1–9
FAOSTAT. 2008. Available at: http://faostat.fao.org/
Gautam S, Pandey SN. 2008. Growth and biochemical responses of nickel toxicity on leguminous crop (Lens esculantum) grown in alluvial soil. Res. Environ. Life Sci. 1: 25–28
Gong M, Chen SN, Song YQ, Li ZG. 1997. Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Aust. J. Plant Physiol. 24: 371–379
Hirschi KD. 2004. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 136: 2438–2442
Hodges DM, Delong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611
Hu X, Jiang M, Zhang J, Zhang A, Lin F, Tan M. 2007. Calcium-calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytol. 173: 27–38
Jiang Y, Huang B. 2001. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J. Exp. Bot. 52: 341–349
Kim Y, Yang YY, Lee Y. 2002. Pb and Cd uptake in rice roots. Physiol. Plant. 116: 368–372
Kiran Y, Sahin A. 2006. The effects of cadmium on seed germination, root development and mitotic of root tip cells of lentil (Lens culinaris Medik.). World J. Agric. Sci. 2: 196–200
Lechowski Z, Bialczyk J. 1993. Calcium mediated cytokinin action on chlorophyll synthesis in isolated embryo of Scots pine. Biol. Plant. 35: 53–62
Mandal SM, Bhattacharyya R. 2012. Rhizobium-Legume symbiosis: A model system for the recovery of metal-contaminated agricultural land, In A Zaidi, PA Wani, MS Khan, eds, Toxicity of heavy metals in legumes and bioremediation, Springer-Verlag Wien, pp 115–118
Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405–410
Mobin M, Khan NA. 2007. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 164: 601–610
Muehlbauer FJ, Cho S, Sarker S, McPhee KE, Coyne CJ, Rajesh PN, Ford R. 2006. Application of biotechnology in breeding lentil for resistance to biotic and abiotic stress. Euphytica 147: 149–156
Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol. 22: 867–880
Noctor G, Foyer CH. 1998. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 49: 249–279
Ortega-Villasante C, Rellán-Álvarez ZZ, Del Campo FF, Carpena-Ruiz RO, Hernández LE. 2005. Cellular damage induced by Cd and mercury in Medicago sativa. J. Exp. Bot. 56: 2239–2251
Ouzounidou G, Moustakas M, Eleftherious EP. 1997. Physiological and ultrastructural effects of cadmium on wheat (Triticum aestivum L.) leaves. Arch. Environ. Contam. Toxicol. 32: 154–160
Rentel MC, Knight MR. 2004. Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 135: 1471–1479
Rivetta A, Negrini N, Cocucci M. 1997. Involvement of Ca 2+-calmodulin in Cd 2+ toxicity during early phases of radish (Raphanus sativus L.) seed germination. Plant Cell Environ. 20: 600–608
Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, del Río LA, Sandalio LM. 2009. Cellular responses of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 150: 229–243
Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Rio LA, Sandalio LM. 2004. Cd-induced subcellular accumulation of O2- and H2O2 in pea leaves. Plant Cell Environ. 27: 1122–1134
Roy BK, Prasad R, Gunjan. 2010. Heavy metal accumulation and changes in metabolic parameters in Cajanas cajan grown in mine spoil. J. Environ. Biol. 31: 567–573
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Rio LA. 2001. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 52: 2115–2126
Sanita di Toppi L, Gabbrielli R. 1999. Response to cadmium in higher plants. Environ. Exp. Bot. 41: 105–130
Schützendübel A, Polle A. 2002. Plant responses to abiotic stresses: heavy metal- induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53: 1351–1365
Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM. 2012. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int. J. Mol. Sci. 13: 6604–6619
Suzuki N. 2005. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotechnol. 22: 19–25
Talukdar D. 2011a. The aneuploid switch: Extra-chromosomal effect on antioxidant defense through trisomic shift in Lathyrus sativus L. Ind. J. Fund. Appl. Life Sci. 1: 263–273
Talukdar D. 2011b. Isolation and characterization of NaCltolerant mutations in two important legumes, Clitoria ternatea L. and Lathyrus sativus L.: Induced mutagenesis and selection by salt stress. J. Med. Plants Res. 5: 3619–3628
Talukdar D. 2011c. Flower and pod production, abortion, leaf injury, yield and seed neurotoxin levels in stable dwarf mutant lines of grass pea (Lathyrus sativus L.) differing in salt stress responses. Int. J. Curr. Res. 2: 46–54
Talukdar D. 2012a. Flavonoid-deficient mutants in grass pea (Lathyrus sativus L.): Genetic control, linkage relationships, and mapping with aconitase and S nitrosoglutathione reductase isozyme loci. The Scientific World Journal 2012: Article ID 345983, 11 pages, doi:1 0.1100/2012/345983
Talukdar D. 2012b. Ascorbate deficient semi-dwarf asfL1 mutant of Lathyrus sativus exhibits alterations in antioxidant defense. Biol. Plant. 56: 675–682
Talukdar D. 2012c. An induced glutathione deficient mutant in grass pea (Lathyrus sativus L.): Modifications in plant morphology, alterations in antioxidant activities and increased sensitivity to cadmium. Biorem. Biodiv. Bioavail. 6: 75–86
Talukdar D. 2012d. Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol. Mol. Biol. Plants DOI:10.1007/s12298-012-0140-8.
Talukdar D. 2013. Bioaccumulation and transport of arsenic in different genotypes of lentil (Lens culinaris Medik.). International Journal of Pharma and Bio Sciences 4: (B) 694–701
Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, Liang J, Yang X. 2011. Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 84: 63–69
Uraguchi S, Fujiwara T. 2012. Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice 5: 5
Vernoux T, Wilson RC, Seeley KA, Reichheld J-P, Muroy S, et al.2000. The ROOT MERISTEMLESS/CADMIUM SENSITIVE 2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–109
Wagner GJ. 1993. Accumulation of cadmium in crop plants and its consequences to human health. Adv. Agron. 51: 173–212
Wang CQ, Chen M, Wang BS. 2007. Betacyanin accumulation in the leaves of C3 halophyte Suaeda salsa L. is induced by watering roots with H2O2. Plant Sci. 172: 1–7
Wang CQ, Song H. 2009. Calcium protects Trifolium repens L. seedlings against cadmium stress. Plant Cell Rep. 28: 1341–1349
Williams PC, Bhatty RS, Deshpande SS, Hussein LA, Savage GP. 1994. Improving nutritional quality of cool season food legumes. In FJ Muehlbauer, WJ Kaiser, eds, Expanding the Production and Use of Cool Season Food Legumes, Kluwer Academic Publishers, Dordrecht, pp 113–129
Yang T, Poovaiah BW. 2003. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 8: 505–512
Yau SK, Erskine W. 2000. Diversity of boron-toxicity tolerance in lentil growth and yield. Genet. Res. Crop Evol. 47: 55–61
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talukdar, D. Exogenous calcium alleviates the impact of cadmium-induced oxidative stress in Lens culinaris medic. Seedlings through modulation of antioxidant enzyme activities. J. Crop Sci. Biotechnol. 15, 325–334 (2012). https://doi.org/10.1007/s12892-012-0065-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-012-0065-3