Abstract
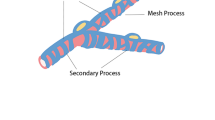
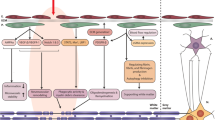
Pericytes are functional components of the neurovascular unit (NVU). They provide support to other NVU components and maintain normal physiological functions of the blood-brain barrier (BBB). The brain ischemia and reperfusion result in pathological alterations in pericytes. The intimate anatomical and functional interactions between pericytes and other NVU components play pivotal roles in the progression of stroke pathology. In this review, we depict the biology and functions of pericytes in the normal brain and discuss their effects in brain injury and repair after ischemia/reperfusion. Since ischemic stroke occurs mostly in elderly people, we also review age-related changes in pericytes and how these changes predispose aged brains to ischemic/reperfusion injury. Strategies targeting pericyte responses after ischemia and reperfusion may provide new therapies for ischemic stroke.



Similar content being viewed by others
References
Li Q, Khatibi N, Zhang JH. Vascular neural network: the importance of vein drainage in stroke. Translational stroke research. 2014;5(2):163–6. doi:10.1007/s12975-014-0335-0.
Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Translational stroke research. 2016;7(4):294–302. doi:10.1007/s12975-016-0469-3.
Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Translational stroke research. 2014;5(4):442–53. doi:10.1007/s12975-014-0336-z.
Lapchak PA. Critical early thrombolytic and endovascular reperfusion therapy for acute ischemic stroke victims: a call for adjunct neuroprotection. Translational stroke research. 2015;6(5):345–54. doi:10.1007/s12975-015-0419-5.
Posada-Duque RA, Barreto GE, Cardona-Gomez GP. Protection after stroke: cellular effectors of neurovascular unit integrity. Front Cell Neurosci. 2014;8:231. doi:10.3389/fncel.2014.00231.
Fisher M. Pericyte signaling in the neurovascular unit. Stroke; a journal of cerebral circulation. 2009;40(3 Suppl):S13–5. doi:10.1161/strokeaha.108.533117.
Liu S, Agalliu D, Yu C, Fisher M. The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des. 2012;18(25):3653–62.
Hill J, Rom S, Ramirez SH, Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol. 2014;9(5):591–605. doi:10.1007/s11481-014-9557-x.
Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab. 2012;32(10):1841–52. doi:10.1038/jcbfm.2012.113.
Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–405. doi:10.1038/nn.2946.
Lange S, Trost A, Tempfer H, Bauer HC, Bauer H, Rohde E, et al. Brain pericyte plasticity as a potential drug target in CNS repair. Drug Discov Today. 2013;18(9–10):456–63. doi:10.1016/j.drudis.2012.12.007.
Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, et al. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood. 2006;108(6):1877–86. doi:10.1182/blood-2006-04-014894.
Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, et al. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1989;37(3):315–21.
Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–5. doi:10.1177/0271678x15610340.
Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–6. doi:10.1038/nature09513.
Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24(7):909–69.
Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169(1):1–11.
ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15(4):6453–74. doi:10.3390/ijms15046453.
Shimizu F, Sano Y, Saito K, Abe MA, Maeda T, Haruki H, et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res. 2012;37(2):401–9. doi:10.1007/s11064-011-0626-8.
Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through tie-2 activation in vitro. J Neurochem. 2004;89(2):503–13. doi:10.1111/j.1471-4159.2004.02343.x.
Kim JH, Kim JH, Yu YS, Kim DH, Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009;87(3):653–9. doi:10.1002/jnr.21884.
Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Laboratory investigation; a journal of technical methods and pathology. 2002;82(4):387–401.
Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol. 2007;83(3):140–8. doi:10.1016/j.pneurobio.2007.07.006.
Badaut J, Bix GJ. Vascular neural network phenotypic transformation after traumatic injury: potential role in long-term sequelae. Translational stroke research. 2014;5(3):394–406. doi:10.1007/s12975-013-0304-z.
Gundersen GA, Vindedal GF, Skare O, Nagelhus EA. Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes. Brain Struct Funct. 2014;219(6):2181–6. doi:10.1007/s00429-013-0629-0.
Patel JP, Frey BN. Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural plasticity. 2015;2015:708306. doi:10.1155/2015/708306.
Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 2014;1550:1–8. doi:10.1016/j.brainres.2014.01.004.
Tu Z, Li Y, Smith DS, Sheibani N, Huang S, Kern T, et al. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52(12):9005–10. doi:10.1167/iovs.11-8008.
Li Q, Chen Y, Li B, Luo C, Zuo S, Liu X, et al. Hemoglobin induced NO/cGMP suppression deteriorate microcirculation via pericyte phenotype transformation after subarachnoid hemorrhage in rats. Scientific reports. 2016;6:22070. doi:10.1038/srep22070.
Sugiyama T. Role of P2X7 receptors in the development of diabetic retinopathy. World J Diabetes. 2014;5(2):141–5. doi:10.4239/wjd.v5.i2.141.
Muramatsu R, Yamashita T. Pericyte function in the physiological central nervous system. Neurosci Res. 2014;81-82:38–41. doi:10.1016/j.neures.2014.01.007.
Nakata M, Nakagomi T, Maeda M, Nakano-Doi A, Momota Y, Matsuyama T. Induction of perivascular neural stem cells and possible contribution to neurogenesis following transient brain ischemia/reperfusion injury. Translational stroke research. 2016; doi:10.1007/s12975-016-0479-1.
Hurtado-Alvarado G, Cabanas-Morales AM, Gomez-Gonzalez B. Pericytes: brain-immune interface modulators. Front Integr Neurosci. 2014;7:80. doi:10.3389/fnint.2013.00080.
Jiang Q, Ewing JR, Chopp M. MRI of blood-brain barrier permeability in cerebral ischemia. Translational stroke research. 2012;3(1):56–64. doi:10.1007/s12975-011-0133-.
Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 2015; doi:10.1016/j.pneurobio.2015.09.008.
Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31(2):693–705. doi:10.1038/jcbfm.2010.148.
Itoh Y, Toriumi H, Yamada S, Hoshino H, Suzuki N. Astrocytes and pericytes cooperatively maintain a capillary-like structure composed of endothelial cells on gel matrix. Brain Res. 2011;1406:74–83. doi:10.1016/j.brainres.2011.06.039.
Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71(5):782–97. doi:10.1016/j.neuron.2011.08.009.
Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–4. doi:10.1038/nature05193.
Filosa JA, Nelson MT, Gonzalez Bosc LV. Activity-dependent NFATc3 nuclear accumulation in pericytes from cortical parenchymal microvessels. American journal of physiology Cell physiology. 2007;293(6):C1797–805. doi:10.1152/ajpcell.00554.2006.
Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. doi:10.1038/ncomms4413.
Tagami M, Kubota A, Nara Y, Yamori Y. Detailed disease processes of cerebral pericytes and astrocytes in stroke-prone SHR. Clinical and experimental hypertension Part A, Theory and practice. 1991;13(5):1069–75.
Poittevin M, Lozeron P, Hilal R, Levy BI, Merkulova-Rainon T, Kubis N. Smooth muscle cell phenotypic switching in stroke. Translational stroke research. 2014;5(3):377–84. doi:10.1007/s12975-013-0306-x.
Lai CH, Kuo KH. The critical component to establish in vitro BBB model: pericyte. Brain Res Brain Res Rev. 2005;50(2):258–65. doi:10.1016/j.brainresrev.2005.07.004.
Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53(6):637–44.
Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci U S A. 2003;100(26):15859–64. doi:10.1073/pnas.2136855100.
Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44(11):4994–5005.
Schrodl F, Trost A, Strohmaier C, Bogner B, Runge C, Kaser-Eichberger A, et al. Rat choroidal pericytes as a target of the autonomic nervous system. Cell Tissue Res. 2014;356(1):1–8. doi:10.1007/s00441-013-1769-5.
Kawamura H, Oku H, Li Q, Sakagami K, Puro DG. Endothelin-induced changes in the physiology of retinal pericytes. Invest Ophthalmol Vis Sci. 2002;43(3):882–8.
Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107(51):22290–5. doi:10.1073/pnas.1011321108.
Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87(1):95–110. doi:10.1016/j.neuron.2015.06.001.
Mazzoni J, Cutforth T, Agalliu D. Dissecting the role of smooth muscle cells versus pericytes in regulating cerebral blood flow using in vivo optical imaging. Neuron. 2015;87(1):4–6. doi:10.1016/j.neuron.2015.06.024.
Arimura K, Ago T, Kamouchi M, Nakamura K, Ishitsuka K, Kuroda J, et al. PDGF receptor beta signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9(1):1–9.
Li Q, Puro DG. Adenosine activates ATP-sensitive K(+) currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res. 2001;907(1–2):93–9.
Matsugi T, Chen Q, Anderson DR. Adenosine-induced relaxation of cultured bovine retinal pericytes. Invest Ophthalmol Vis Sci. 1997;38(13):2695–701.
Haefliger IO, Anderson DR. Oxygen modulation of guanylate cyclase-mediated retinal pericyte relaxations with 3-morpholino-sydnonimine and atrial natriuretic peptide. Invest Ophthalmol Vis Sci. 1997;38(8):1563–8.
Haefliger IO, Chen Q, Anderson DR. Effect of oxygen on relaxation of retinal pericytes by sodium nitroprusside. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1997;235(6):388–92.
Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15(9):1031–7. doi:10.1038/nm.2022.
Shojaee N, Patton WF, Hechtman HB, Shepro D. Myosin translocation in retinal pericytes during free-radical induced apoptosis. J Cell Biochem. 1999;75(1):118–29.
Pennypacker KR. Peripheral immune response to CNS injury. Translational stroke research. 2012;3(3):305. doi:10.1007/s12975-012-0204-7.
Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Translational stroke research. 2014;5(5):543–53. doi:10.1007/s12975-014-0349-7.
Olson LE, Soriano P. PDGFRbeta signaling regulates mural cell plasticity and inhibits fat development. Dev Cell. 2011;20(6):815–26. doi:10.1016/j.devcel.2011.04.019.
Lyck R, Enzmann G. The physiological roles of ICAM-1 and ICAM-2 in neutrophil migration into tissues. Curr Opin Hematol. 2015;22(1):53–9. doi:10.1097/moh.0000000000000103.
Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209(6):1219–34. doi:10.1084/jem.20111622.
Katare RG, Madeddu P. Pericytes from human veins for treatment of myocardial ischemia. Trends in cardiovascular medicine. 2013;23(3):66–70. doi:10.1016/j.tcm.2012.09.002.
Jeynes B. Reactions of granular pericytes in a rabbit cerebrovascular ischemia model. Stroke; a journal of cerebral circulation. 1985;16(1):121–5.
Sakuma R, Kawahara M, Nakano-Doi A, Takahashi A, Tanaka Y, Narita A, et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation. 2016;13(1):57. doi:10.1186/s12974-016-0523-9.
Ozen I, Deierborg T, Miharada K, Padel T, Englund E, Genove G, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128(3):381–96. doi:10.1007/s00401-014-1295-x.
Duz B, Oztas E, Erginay T, Erdogan E, Gonul E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology. 2007;55(3):279–84. doi:10.1016/j.cryobiol.2007.08.009.
Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc Res. 2002;64(1):116–9. doi:10.1006/mvre.2002.2413.
Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston Jr LL, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke; a journal of cerebral circulation. 2004;35(4):998–1004. doi:10.1161/01.str.0000119383.76447.05.
Takata F, Dohgu S, Matsumoto J, Takahashi H, Machida T, Wakigawa T, et al. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J Neuroinflammation. 2011;8:106. doi:10.1186/1742-2094-8-106.
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–61. doi:10.1038/nature09522.
Willard AL, Herman IM. Vascular complications and diabetes: current therapies and future challenges. J Ophthalmol. 2012;2012:209538. doi:10.1155/2012/209538.
Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, et al. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res. 2012;83(3):352–9. doi:10.1016/j.mvr.2012.02.009.
Li H, Gao A, Feng D, Wang Y, Zhang L, Cui Y, et al. Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood-brain barrier integrity during experimental cerebral ischemia-reperfusion injury. Translational stroke research. 2014;5(5):618–26. doi:10.1007/s12975-014-0354-x.
Wang YL, Hui YN, Guo B, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye (London, England). 2007;21(12):1501–10. doi:10.1038/sj.eye.6702716.
Zechariah A, ElAli A, Doeppner TR, Jin F, Hasan MR, Helfrich I, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke; a journal of cerebral circulation. 2013;44(6):1690–7. doi:10.1161/strokeaha.111.000240.
Kandadai MA, Meunier JM, Hart K, Holland CK, Shaw GJ. Plasmin-loaded echogenic liposomes for ultrasound-mediated thrombolysis. Translational stroke research. 2015;6(1):78–87. doi:10.1007/s12975-014-0376-4.
Li Z, Wang J, Zhao C, Ren K, Xia Z, Yu H, et al. Acute blockage of notch signaling by DAPT induces neuroprotection and neurogenesis in the neonatal rat brain after stroke. Translational stroke research. 2016;7(2):132–40. doi:10.1007/s12975-015-0441-7.
Nomura M, Yamagishi S, Harada S, Hayashi Y, Yamashima T, Yamashita J, et al. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270(47):28316–24.
Yonekura H, Sakurai S, Liu X, Migita H, Wang H, Yamagishi S, et al. Placenta growth factor and vascular endothelial growth factor B and C expression in microvascular endothelial cells and pericytes. Implication in autocrine and paracrine regulation of angiogenesis. J Biol Chem. 1999;274(49):35172–8.
Yamagishi S, Yonekura H, Yamamoto Y, Fujimori H, Sakurai S, Tanaka N, et al. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Laboratory investigation; a journal of technical methods and pathology. 1999;79(4):501–9.
Takagi H, King GL, Aiello LP. Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes. 1996;45(8):1016–23.
Park YS, Kim NH, Jo I. Hypoxia and vascular endothelial growth factor acutely up-regulate angiopoietin-1 and Tie2 mRNA in bovine retinal pericytes. Microvasc Res. 2003;65(2):125–31.
Chakroborty D, Sarkar C, Yu H, Wang J, Liu Z, Dasgupta PS, et al. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci U S A. 2011;108(51):20730–5. doi:10.1073/pnas.1108696108.
Feng Y, vom Hagen F, Pfister F, Djokic S, Hoffmann S, Back W, et al. Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost. 2007;97(1):99–108.
Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105(3):1094–101. doi:10.1182/blood-2004-06-2315.
Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development (Cambridge, England). 2014;141(2):307–17. doi:10.1242/dev.096107.
Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7(3):269–76. doi:10.1007/s10456-004-4182-6.
Minami Y, Sasaki T, Bochimoto H, Kawabe J, Endo S, Hira Y, et al. Prostaglandin I2 analog suppresses lung metastasis by recruiting pericytes in tumor angiogenesis. Int J Oncol. 2015;46(2):548–54. doi:10.3892/ijo.2014.2783.
Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26(4):545–55. doi:10.1038/sj.jcbfm.9600214.
Piquer-Gil M, Garcia-Verdugo JM, Zipancic I, Sanchez MJ, Alvarez-Dolado M. Cell fusion contributes to pericyte formation after stroke. J Cereb Blood Flow Metab. 2009;29(3):480–5. doi:10.1038/jcbfm.2008.150.
Kane R, Godson C, O’Brien C. Chordin-like 1, a bone morphogenetic protein-4 antagonist, is upregulated by hypoxia in human retinal pericytes and plays a role in regulating angiogenesis. Mol Vis. 2008;14:1138–48.
Matsuki M, Kabara M, Saito Y, Shimamura K, Minoshima A, Nishimura M, et al. Ninjurin1 is a novel factor to regulate angiogenesis through the function of pericytes. Circulation journal: official journal of the Japanese Circulation Society. 2015;79(6):1363–71. doi:10.1253/circj.CJ-14-1376.
Wu P, Yonekura H, Li H, Nozaki I, Tomono Y, Naito I, et al. Hypoxia down-regulates endostatin production by human microvascular endothelial cells and pericytes. Biochem Biophys Res Commun. 2001;288(5):1149–54. doi:10.1006/bbrc.2001.5903.
Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem cells (Dayton, Ohio). 2015;33(6):1962–74. doi:10.1002/stem.1977.
Amos PJ, Mulvey CL, Seaman SA, Walpole J, Degen KE, Shang H, et al. Hypoxic culture and in vivo inflammatory environments affect the assumption of pericyte characteristics by human adipose and bone marrow progenitor cells. American journal of physiology Cell physiology. 2011;301(6):C1378–88. doi:10.1152/ajpcell.00460.2010.
Karow M, Schichor C, Beckervordersandforth R, Berninger B. Lineage-reprogramming of pericyte-derived cells of the adult human brain into induced neurons. Journal of visualized experiments: JoVE. 2014;87 doi:10.3791/51433.
Nakagomi T, Nakano-Doi A, Kawamura M, Matsuyama T. Do vascular pericytes contribute to neurovasculogenesis in the central nervous system as multipotent vascular stem cells? Stem Cells Dev 2015; 24(15):1730–1739. doi:10.1089/scd.2015.0039.
Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333(6039):238–42.
Okada S, Nakamura M, Mikami Y, Shimazaki T, Mihara M, Ohsugi Y, et al. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 2004;76(2):265–76. doi:10.1002/jnr.20044.
Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233(2):717–32.
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–27. doi:10.1016/j.neuron.2010.09.043.
Dardick I, Hammar SP, Scheithauer BW. Ultrastructural spectrum of hemangiopericytoma: a comparative study of fetal, adult, and neoplastic pericytes. Ultrastruct Pathol. 1989;13(2–3):111–54.
Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991;229(3):384–98. doi:10.1002/ar.1092290311.
Peinado MA, Quesada A, Pedrosa JA, Torres MI, Martinez M, Esteban FJ, et al. Quantitative and ultrastructural changes in glia and pericytes in the parietal cortex of the aging rat. Microsc Res Tech. 1998;43(1):34–42. doi:10.1002/(sici)1097-0029(19981001)43:1<34::aid-jemt6>3.0.co;2-g.
Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging. 2006;27(12):1838–47. doi:10.1016/j.neurobiolaging.2005.10.021.
Soltanpour N, Baker DM, Santer RM. Neurons and microvessels of the nodose (vagal sensory) ganglion in young adult and aged rats: morphometric and enzyme histochemical studies. Tissue & cell. 1996;28(5):593–602.
Alba C, Vidal L, Diaz F, Villena A, de Vargas IP. Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Res Bull. 2004;64(2):145–53. doi:10.1016/j.brainresbull.2004.06.006.
Rensink AA, Otte-Holler I, de Boer R, Bosch RR, ten Donkelaar HJ, de Waal RM, et al. Insulin inhibits amyloid beta-induced cell death in cultured human brain pericytes. Neurobiol Aging. 2004;25(1):93–103.
Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, et al. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1561–7. doi:10.1161/atvbaha.112.300749.
Kobayashi T, Puro DG. Loss of insulin-mediated vasoprotection: early effect of diabetes on pericyte-containing microvessels of the retina. Invest Ophthalmol Vis Sci. 2007;48(5):2350–5. doi:10.1167/iovs.06-1357.
Omote Y, Deguchi K, Kono S, Liu N, Liu W, Kurata T, et al. Neurovascular protection of cilostazol in stroke-prone spontaneous hypertensive rats associated with angiogenesis and pericyte proliferation. J Neurosci Res. 2014;92(3):369–74. doi:10.1002/jnr.23327.
Deguchi K, Liu N, Liu W, Omote Y, Kono S, Yunoki T, et al. Pericyte protection by edaravone after tissue plasminogen activator treatment in rat cerebral ischemia. J Neurosci Res. 2014;92(11):1509–19. doi:10.1002/jnr.23420.
Shaikh H, Lechpammer M, Jensen FE, Warfield SK, Hansen AH, Kosaras B, et al. Increased brain perfusion persists over the first month of life in term asphyxiated newborns treated with hypothermia: does it reflect activated angiogenesis? Translational stroke research. 2015;6(3):224–33. doi:10.1007/s12975-015-0387-9.
Youn SW, Jung KH, Chu K, Lee JY, Lee ST, Bahn JJ, et al. Feasibility and safety of intra-arterial pericyte progenitor cell delivery following mannitol-induced transient blood-brain barrier opening in a canine model. Cell Transplant. 2015;24(8):1469–79. doi:10.3727/096368914x682413.
Acknowledgments
This work was supported by the NIH/National Institute of Neurological Disorders and Stroke (NINDS) grants NS094573 and NS092618 (to X.H.) and NS095671 and NS089534 (to J.C.) and the American Heart Association (13SDCG14570025 to X.H.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors claim no conflict of interest.
Rights and permissions
About this article
Cite this article
Cai, W., Liu, H., Zhao, J. et al. Pericytes in Brain Injury and Repair After Ischemic Stroke. Transl. Stroke Res. 8, 107–121 (2017). https://doi.org/10.1007/s12975-016-0504-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0504-4